Hierarchical NiO hollow microspheres assembled from nanosheet-stacked nanoparticles and their application in a gas sensor†
Guoxing
Zhu
a,
Chunyan
Xi
a,
Huan
Xu
a,
Dan
Zheng
a,
Yuanjun
Liu
b,
Xiang
Xu
a and
Xiaoping
Shen
*a
aSchool of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang, 212013, China. E-mail: xiaopingshen@163.com; Fax: (+86)511-84401889; Tel: (+86)511-84401889
bSchool of Biology and Chemical Engineering, Jiangsu University of Science and Technology, Zhenjiang, 212013, China
First published on 28th February 2012
Abstract
A facile and robust route for the mass preparation of hollow NiO microspheres assembled from nanosheet-stacked nanoparticles is developed. The Ni(HCO3)2 precursor with a hollow spherical structure was firstly prepared by a hydrothermal reaction without any surfactants or organic additives. The reaction generated gas bubble may act as a template for the formation of Ni(HCO3)2 hollow microspheres. These are converted into hierarchical NiO hollow microspheres assembled from nanoparticles (with diameter of ∼25 nm) upon calcination, which are further assembled by the stacking of ultrathin nanosheets. The hierarchical NiO hollow structures are attractive for catalyst, sensor and environmental applications, benefiting from their large surfaces. The sensing properties of the hierarchical NiO hollow microspheres were evaluated. They show high sensitivity, short response and recovery times, and good response and recovery characteristics to n-butanol. As compared with NiO nanoparticles with similar dimensions (∼20–35 nm), the nanoparticle assembled NiO hollow microspheres exhibit enhanced gas sensing properties. The effective integration of several nanostructures in one microunit would provide a novel way to design new materials for nanodevices.
Introduction
Nanosheets are a new class of two-dimensional (2D) nanostructure that are characterized by a thickness on the level of nanometres (often smaller than 5 nm) and lateral dimensions up to the micrometre scale. Since the discovery of graphene, such two-dimensional nanostructures have attracted great attention in the past few years.1–3 They are fascinating not only owing to their unique structure and structure-induced properties but also to their numerous promising applications.4,5 These 2D nanosheets offer very interesting features. First, in contrast to other low dimensional nanostructures, the 2D structure facilitates their processability. They can be assembled into highly oriented films by employing Langmuir–Blodgett (LB) or layer-by-layer (LbL) techniques, which offer the possibility to precisely control the thickness of the formed films.6–8 Furthermore, the ability of the nanosheets to self-assemble allows the formation of restacked lamellar aggregates with a disordered and porous nature,9 leading to enhanced properties as found in H4Nb6O1710 and HTiNbO511 for photocatalysis, Li/MnO212 for electrodes of lithium-ion batteries, and H/RuO2 for double-layer capacitors.13 Second, based on these 2D nanosheets, multifunctional hybrid structures with improved physical properties may be constructed through rational design and choice of constituting nanoblocks and control of their arrangement in the solid state.14,15 So far, some transition-metal oxide nanosheets such as Ca2Nb3O10,16 CeO2,17 Nb6O17,18 Ti1−δO2,19,20 and MnO221,22 have been investigated due to the rich chemistry and physics of their bulk counterparts. However, the present transition-metal oxide nanosheets are only limited to several materials, mostly layered compounds. Moreover, most research has focused on the preparation of nanosheets, limited reports have been on their assembly.Among the oxide materials, we are particularly interested in NiO, which is an important p-type semiconductor with a cubic structure. It has been extensively investigated because of its myriad applications in catalysts,23 gas sensors,24,25 battery materials,26 electrochromic coatings, active optical fibers, fuel cell electrodes,27 and so on. NiO is also used in magnetic multilayer devices,28 and has potential applications in nonvolatile memories due to its excellent resistive switching behavior.29,30 Up to now, a variety of methods have been used to achieve nanostructured NiO, such as hollow spheres,31,32 nanorods/wires,33–35 nanoflowers,36 polyhedrons,37 mesoporous NiO walls,38 and palates.39
In this study, we present a new hierarchical NiO hollow structure obtained by a facile method, that is, ultrathin NiO nanosheets are assembled layer-by-layer into nanoparticles, which then further aggregated into hollow microspheres. The route involves the controlled precipitation of Ni(HCO3)2 through a hydrothermal process followed by calcination. This simple, salt dicarbonate-mediated method may be utilized to fabricate a wide variety of nanosheet-based hierarchical nanoarchitectures. Furthermore, we show that the as-synthesized NiO nanostructure has enhanced gas sensing performance as compared with NiO nanoparticles of similar size.
Experimental
Preparation of hierarchical NiO hollow microspheres
All the reagents of analytical grade (A. R.) were purchased from Shanghai Chemical Reagent Co., Ltd. and used without further purification. In a typical experimental procedure, 2.8 g of Ni(NO3)2·6H2O was dissolved in 25 mL of deionized water. The solution was then added to 3.5 g of urea with stirring. After complete dissolution, the obtained solution was then transferred into an autoclave. The subsequent hydrothermal reaction was conducted in an electric oven at 120 °C for 5 h. The autoclave was then allowed to cool down naturally to room temperature; the as-obtained solid precursor was separated by centrifugation and thoroughly washed with ethanol and deionized water five times. For the generation of nanostructured NiO, an appropriate amount of the as-synthesized precursor was calcined in air at 500 °C for 1 h. After cooling down naturally to room temperature, hierarchical NiO hollow microspheres were obtained.Control experiment for the preparation of NiO nanoparticles
For comparison of gas sensing properties, NiO nanoparticles were prepared. In a typical procedure, 0.94 g of NiCl2·6H2O was dissolved in 32 mL of ethylene glycol. The solution was then added to 2.8 g of CH3COONa and 0.75 mL of polyethylene glycol (PEG-400) with stirring, which were then transferred to an autoclave, and the reaction was conducted in an electric oven at 180 °C for 8 h. After cooling naturally to room temperature, the as-obtained solid was separated by centrifugation and thoroughly washed with ethanol and deionized water. NiO nanoparticles were generated by the subsequent calcination of the obtained solid in air at 500 °C for 1 h.Characterization
For crystal phase identification, the samples were examined with a X-ray powder diffractometer (XRD; Cu-Kα radiation; Shimaduzu; λ = 1.5406 Å). The morphologies and dimensions of the samples were investigated using a Hitachi S-4800 field emission scanning electron microscope (FE-SEM) and a JEOL-2100 high-resolution transmission electron microscope (HR-TEM), which was also used for selected area electron diffraction (SAED) analysis (accelerating voltage of 200 kV). Raman spectra were recorded with a JY HR-800 Raman spectrometer with a 453 nm laser excitation. The gas sensing tests were carried out on a commercial HW-30 gas sensing measurement system (HanWei Electronics Co., Ltd, Henan, China) at a relative humidity of 20–35%. The structure, fabrication, and testing principle of our gas sensor based on the as-prepared NiO are similar to those of our reported In2O3 sensor.40 The sensing response, S, was determined as the ratio, Rg/Ra, where Ra is the resistance in ambient air and Rg is the resistance in the tested gas atmosphere.Results and discussion
The crystal structure and purity of the as-synthesized precursor sample were firstly examined by powder XRD. As shown in Fig. 1a, all the peaks observed can be easily indexed to Ni(HCO3)2 with body-centered cubic structure (JCPDS No. 1507-82). No peaks corresponding to other substances such as Ni(OH)2 or Ni2(OH)2CO3 were observed. (Fig. 1a). After thermal treatment of the as-obtained Ni(HCO3)2 precursor sample at 500 °C in air for 1 h, face-centered cubic NiO (JCPDS No. 78-0643) is generated, as indicated by the XRD pattern shown in Fig. 1b, in which the peaks correspond to the (111), (200), (220), (311) and (222) crystal planes of face-centered cubic NiO. It can be concluded that there has been complete conversion of Ni(HCO3)2 into NiO during the calcination process since no peaks other than those of NiO are detected in the XRD pattern. The sharp diffraction peaks suggest the high crystallinity of NiO. | ||
| Fig. 1 XRD patterns of the as-prepared (a) Ni(HCO3)2 precursor and (b) NiO product; the standard data of cubic Ni(HCO3)2 (JCPDS No. 1507-82) are also shown for comparison. | ||
The size and morphology of the precursor product were examined by FE-SEM. As shown in Fig. 2a–b, the SEM images of Ni(HCO3)2 show nearly spherical units with diameter of 0.5–1.5 μm. A hole can be observed in some Ni(HCO3)2 sub-microspheres (as shown by arrows in Fig. 2a), which suggests the hollow structure feature.
 | ||
| Fig. 2 (a, b) SEM images of the as-synthesized Ni(HCO3)2. | ||
The hydrothermal reaction of transition metal ions and urea often produces carbonate hydroxide salts, which are often used as precursors for the fabrication of oxide micro-/nanostructures.41,42 Here nickel dicarbonate is formed. It was found that which salt is formed is dependent on the concentration of urea. A lower concentration of urea would cause the formation of carbonate hydroxide salts. The formation of Ni(HCO3)2 could be described by the following reactions:
| CO(NH2)2 + 3H2O → 2NH3·H2O + CO2 | (1) |
| NH3·H2O → NH4+ + OH− | (2) |
| CO2 + 2OH− → CO32− + H2O | (3) |
| CO32− + H2O + CO2 → 2HCO3− | (4) |
| Ni2+ + 2HCO3− → Ni(HCO3)2 | (5) |
When the precursor solution is heated to 120 °C, urea undergoes hydrolysis and releases OH− through reactions (1) and (2). The generated CO2 will transform into CO32− ions in alkali solution according to reaction (3). When the concentration of urea is high, the large amount of CO2 produced from reaction (1) would further react with CO32− to form HCO3− ions (reaction (4)), which then react with Ni2+ forming Ni(HCO3)2 monomers (reaction (5)). If the concentration of urea is low, the amount of CO2 will be low, and not enough CO2 will react with CO32− forming HCO3−, so the formed products are carbonate hydroxide salts. As the reaction proceeds, the concentration of Ni(HCO3)2 monomers in the solution will reach saturation, form Ni(HCO3)2 crystal nuclei, and subsequently grow into nanostructures.
It is interesting that the Ni(HCO3)2 crystals grow into hollow structures in such a reaction system without surfactants or additive templates. The formation of the hollow structures would relate with the abundant CO2 bubbles produced from the hydrolysis of urea. After the initial nucleation of the monomers, Ni(HCO3)2 nuclei will grow and have a tendency to aggregate. At the same time, lots of CO2 produced from reaction (1) owing to the high concentration of urea would cause the generation of microbubbles and provide an aggregation center. Driven by the minimization of interfacial energy, the Ni(HCO3)2 may aggregate at the gas–liquid interface between CO2 and solution, finally forming hollow Ni(HCO3)2 microspheres. The use of gas bubbles for the fabrication of hollow microspheres has also been observed for ZnSe.43 Compared with other template methods, using reaction generated gas bubbles as soft-templates to fabricate hollow microspheres has many advantages, such as convenience, no need to remove templates, and avoiding the introduction of impurities, and is therefore suitable for modern chemical synthesis.
To obtain NiO hollow microspheres, the as-synthesized Ni(HCO3)2 products were calcined at 500 °C in air for 1 h. Fig. 3 shows SEM images of the prepared NiO product. It is apparent that the spherical morphology of Ni(HCO3)2 is somewhat preserved, while the size has shrunk from 0.5–1.5 μm to 0.3–1.2 μm. One can see that the surfaces of the NiO microspheres are coarse and some of them have small holes (as shown by the arrows in Fig. 3). It seems that the microspheres are loose and porous, which would relate to the release of water and carbon dioxide during the thermal treatment process.
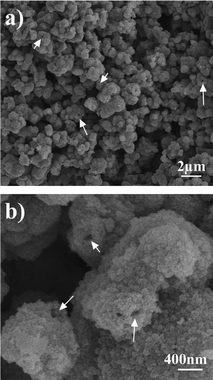 | ||
| Fig. 3 (a, b) SEM images of the as-synthesized NiO hollow microspheres. | ||
Further structural characterization comes from TEM observations. The TEM image displayed in Fig. 4a clearly shows the hollow structural feature. According to the TEM image with higher magnification (Fig. 4b), it is observed that the NiO microspheres are composed of NiO nanoparticles with size around 25 nm. Due to the close aggregation and packing of these NiO nanoparticles, the hollow structure is fabricated. The corresponding SAED pattern (inset of Fig. 4b) recorded on one NiO hollow microsphere suggests its polycrystalline nature. The diffraction rings can be easily indexed to the (311), (222), (220), (111), and (200) lattice planes of cubic NiO. Interestingly, these NiO nanoparticles have further microstructures. Careful observation by HRTEM found that they are actually composed by the stacking of nanosheet-like building blocks, as shown in Fig. 4c. The observation on at least ten NiO nanoparticles confirms the same structural feature (more TEM images are shown in Fig. S1, See ESI†). As shown in the noted area of Fig. 4c, the sheet building blocks are ultrathin with thickness of ∼2 nm. The scheme shown in the inset of Fig. 4c shows the structural feature of these unique NiO units. It is proposed that the formation of these lamellar NiO units is related to the intrinsic crystal structure of Ni(HCO3)2.
 | ||
| Fig. 4 (a–d) TEM and HRTEM images of the as-synthesized NiO hollow microspheres. The inset of (b) is a SAED pattern recorded on one hollow microsphere. The scheme shown in the inset of (c) shows the structural feature of these unique NiO units. The inset of (d) is a SAED pattern recorded on one NiO nanoparticle. | ||
A microstructure analysis of these sheet-like units was also conducted (shown in Fig. 4d). The observed lattice fringe spacing of 0.21 nm is consistent with the d-spacing of the (200) planes in cubic NiO. The inset of Fig. 4d shows a SAED pattern recorded on one NiO nanoparticle with [001] axes. From this the surface lattice planes of {001} are concluded. Owing to the coarse and porous structural feature, the synthesized NiO hollow microspheres fabricated by the aggregation of NiO nanoparticles could be used as sensing materials and as photoanodes for the low-cost manufacture of high-performance solar cells.
Fig. 5 shows the Raman spectrum of the as-synthesized hierarchical NiO hollow microspheres. There are three Raman peaks at 536, 688, and 1057 cm−1. The peak at 536 cm−1 can be assigned to the longitudinal optical (LO) one-phonon (1P) modes of NiO. The peaks at 688 and 1057 cm−1 can be assigned as two-phonon (2P) modes of 2TO and 2LO, respectively.44 Compared with NiO single-crystal, the 1P band is pronounced in our prepared NiO nanostructures due to the presence of defects or surface effects. The relatively weaker band of the 1P mode suggests a low concentration of defects.45 However, the two-magnon band usually located at 1490 cm−1 is undetectable for our sample, which agrees well with the previous report,45 suggesting a decrease of antiferromagnetic spin correlation and the antiferromagnetic-to-paramagnetic transition in the nanosized NiO.
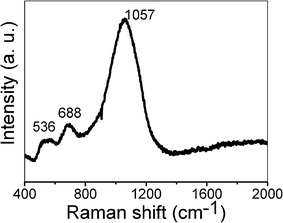 | ||
| Fig. 5 Raman spectrum of the as-synthesized NiO hollow microspheres. | ||
Hollow nanostructures with thin shell layers are very attractive in sensing and catalyst fields due to their high surface area and low density. Enhanced gas responses have been demonstrated with hollow oxide nanostructures as gas sensor materials.46,47 Here, the gas sensing properties of the as-prepared hierarchical NiO hollow microspheres were investigated. For comparison, another gas sensor based on NiO nanoparticles with a diameter of 20∼35 nm was also fabricated and tested (see Experimental section and Fig. S2 in the ESI†).
The sensing properties of the two sensors were measured at a working temperature of 350 °C with air as the reference gas. n-Butanol was used as a model gas for the sensor property tests. The obtained response and recovery characteristic curves against n-butanol concentration (5–800 ppm) of the two sensors are shown in Fig. 6a. At the measured temperature, the sensor resistance increases upon exposure to n-butanol and recovers approximately to the initial value when the test chamber is refreshed with air, which is expected for a p-type semiconductor (such as NiO) upon exposure to reducing gas.48 The results clearly indicate that even the lowest concentration of n-butanol, i.e. 5 ppm, can be detected. The sensing response of the as-synthesized NiO hollow microspheres is higher than that of the NiO nanoparticles. This means that the unique hierarchical hollow nanostructure has a better sensing performance to n-butanol than that of the NiO nanoparticles. After eight cycles between the test gas and fresh air, the resistances of the sensors could recover to their initial state, which indicates that the sensors have good reversibility. For an effective sensor, short response and recovery times are needed in the rapid detection of flammable and toxic gases. For our sensors, the response time and recovery time (defined as the time required to reach 90% of the final equilibrium value) are less than 60 s.
 | ||
| Fig. 6 (a) Response and recovery characteristic curves of the two sensors based on the prepared hierarchical NiO hollow microspheres and NiO nanoparticles to butanol, respectively. (b) The gas concentration-dependent responses. | ||
The sensing responses as a function of n-butanol vapor concentration from 5 ppm to 800 ppm are shown in Fig. 6b. It can be seen that the responses Rg/Ra of the two sensors are dramatically enhanced with the increasing concentration of the test gas at first (below 200 ppm). Above 200 ppm of n-butanol, the responses increase slowly and tend to saturation. This may be attributed to many reasons such as the insufficiency of negatively charged oxygen ions on the sensor which cannot oxidize all the analyte gases near the sensor surface, and the full covering of gas molecules on the sensor.49,50 The response of oxide semiconductor sensor to gas is usually empirically depicted as S = 1+APgβ, where Pg is the target gas partial pressure, and so the response is characterized by the prefactor A and exponent β.51,52 The value of β depends mainly on the charge of the surface species and stoichiometry of the elementary reactions on the surface and usually takes 1 or 1/2. As shown in Fig. 6b, the responses increase exponentially for both sensors, suggesting the value of β is 1/2 in these cases (Fig. S3, See ESI†).
The sensing response properties of the sensors towards other gases, propanol, acetone, formaldehyde, ethanol, heptanes, were also tested. Fig. 7 shows the responses of the two sensors to 100 ppm of test gases. Obviously, the sensor based on NiO hollow microspheres has a higher response than that fabricated from NiO nanoparticles for all tested gases. For the NiO hollow microsphere sensor, the response is highest for butanol, followed by propanol, acetone, formaldehyde, ethanol, and heptane.
 | ||
| Fig. 7 The responses of the two sensors based on the prepared NiO hollow microspheres and NiO nanoparticles to 100 ppm of butanol, propanol, acetone, formaldehyde, ethanol, and heptane. | ||
The change in resistance is primarily caused by the adsorption and desorption of the target gas moleculars on the surface of the sensing material. With air as reference gas, the oxygen molecules in the air are adsorbed on the surface of the NiO (according to eqn (1) to (4)) and capture electrons from them.53 NiO is a typical p-type semiconductor with hole carriers; the adsorption of the oxygen molecules results in an increase in the carrier density and the formation of a hole-rich layer on the surface of NiO; this is in contrast with an n-type semiconductor.
| O2 (gas) ↔ O2 (ads) | (1) |
| O2 (ads) + e− → O2− (ads) | (2) |
| O2− (ads) + e− →2O− (ads) | (3) |
| O− (ads) + e− →O2− (ads) | (4) |
In general, below 150 °C O2− dominates, while above this temperature the atomic species (O2−, O−) are the main forms.54 At the beginning, the air supplies enough oxygen molecules adsorbed on the NiO surface. When n-butanol or a similar reducing gas is injected in the test chamber, the test molecules interact with pre-adsorbed oxygen on the surface of NiO, generating water and carbon dioxide molecules according to equ (5). At the same time, the captured electrons are released, which neutralizes the hole carriers in the NiO surface layer and the sensor resistance increases until a new equilibrium is obtained. When the test chamber is charged with fresh air, oxygen molecules (in the air) will again adsorb on the surface of the NiO and the sensor resistances decrease to their initial values.
| C4H9OH + 12O− (ads) → 4CO2 (gas) + 5H2O (gas) + 12e− | (5) |
The gas sensing superiority of hierarchical NiO hollow spheres over NiO nanoparticles can be understood. Firstly, the hierarchical NiO microspheres with a hollow structure have a relatively larger accessible surface area, thus more gas molecules including O2 and test gas would adsorb on the surface and cause a higher gas sensing response. In contrast, the NiO nanoparticles easily aggregate when the samples are coated on supports for sensor fabrication. Secondly, from theoretical simulations and experimental results, sensor response can remarkably increase as the average crystallite size of the sensing material decreases to about 20 nm, which is about twice the thickness of the electron depletion layer.55,56 The diameter of the NiO building blocks (about 25 nm) in the NiO hollow spheres is near this value, which is obviously beneficial for the enhancement of sensing performance. Thirdly, the unique 2D sheet-like structures would also contribute to the sensing. It is well known that the resistance of a sensing film is mainly controlled by the inter-nanocrystal contact barrier, the modulation of which by gas is also responsible for the sensing response.40,57 A distinct characteristic of the sensor based on our prepared NiO hierarchical structures is that besides the contacts between particles and particles, there are face-to-face contacts between nanosheets. Both of them will contribute to the sensing properties. This is in contrast to the NiO nanoparticles without hierarchical structure.
Conclusions
Large-scale hierarchical NiO hollow microspheres have been successfully synthesized via the Ni(HCO3)2 precursor. The in situ generated CO2 bubbles assist the formation of hollow Ni(HCO3)2 microspheres, which are converted into NiO by calcination. The obtained NiO hollow microspheres are composed of NiO nanoparticles, which are further assembled from ultrathin nanosheets. Enhanced gas sensing properties have been demonstrated to a series of volatile organic compounds. The synthesized NiO hollow microspheres were found to be effective in the detection of butanol, propanol, and acetone. The sensing response is higher than that of NiO nanoparticles with similar size. The present results imply that the inorganic salt precursor route would be an effective way to achieve higher structural control and property improvement for oxide nanostructures.Acknowledgements
The authors are grateful for financial support from the Startup Fund for Distinguished Scholars (No. 10JDG132), China Postdoctoral Science Foundation (2011M500085), Jiangsu Postdoctoral Science Foundation (1102001C) and the National Natural Science Foundation of China (No. 51102117, No. 51072071).References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS.
- R. Z. Ma, K. Takada, K. Fukuda, N. Iyi, Y. Bando and T. Sasaki, Angew. Chem., Int. Ed., 2008, 47, 86 CrossRef CAS.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132 CrossRef CAS.
- C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Angew. Chem., Int. Ed., 2009, 48, 7752 CrossRef CAS.
- P. V. Kamat, J. Phys. Chem. Lett., 2010, 1, 520 CrossRef CAS.
- S. Bai and X. P. Shen, RSC Adv., 2012, 2, 64 RSC.
- Z. Liu, R. Ma, M. Osada, N. Iyi, Y. Ebina, K. Takada and T. Sasaki, J. Am. Chem. Soc., 2006, 128, 4872 CrossRef CAS.
- J. X. He, K. Kobayashi, M. Takahashi, G. Villemure and A. Yamagishi, Thin Solid Films, 2001, 397, 255 CrossRef CAS.
- Y. Ebina, T. Sasaki, M. Harada and M. Watanabe, Chem. Mater., 2002, 14, 4390 CrossRef CAS.
- K. Domen, Y. Ebina, S. Ikeda, A. Tanaka, J. N. Kondo and K. Maruya, Catal. Today, 1996, 28, 167 CrossRef CAS.
- A. Takagaki, M. Sugisawa, D. Lu, J. N. Kondo, M. Hara, K. Domen and S. Hayashi, J. Am. Chem. Soc., 2003, 125, 5479 CrossRef CAS.
- L. Wang, K. Takada, A. Kajiyama, M. Onoda, Y. Michiue, L. Zhang, M. Watanabe and T. Sasaki, Chem. Mater., 2003, 15, 4508 CrossRef CAS.
- W. Sugimoto, H. Iwata, Y. Yasunaga, Y. Murakami and Y. Takasu, Angew. Chem., Int. Ed., 2003, 42, 4092 CrossRef CAS.
- D. M. Kaschak, J. T. Lean, C. C. Waraksa, G. B. Saupe, H. Usami and T. E. Mallouk, J. Am. Chem. Soc., 1999, 121, 3435 CrossRef CAS.
- M. Osada, Y. Ebina, K. Takada and T. Sasaki, Adv. Mater., 2006, 18, 1023 CrossRef CAS.
- M. Fang, C. H. Kim, G. B. Saupe, H.-N. Kim, C. C. Waraksa, T. Miwa, A. Fujishima and T. E. Mallouk, Chem. Mater., 1999, 11, 1526 CrossRef CAS.
- T. Yu, B. Lim and Y. Xia, Angew. Chem., Int. Ed., 2010, 49, 4484 CrossRef CAS.
- R. Abe, K. Shinohara, A. Tanaka, M. Hara, J. N. Kondo and K. Domen, J. Mater. Res., 1998, 13, 861 CrossRef CAS.
- T. Sasaki, M. Watanabe, H. Hashizume, H. Yamada and H. Nakazawa, J. Am. Chem. Soc., 1996, 118, 8329 CrossRef CAS.
- X. H. Yang, Z. Li, G. Liu, J. Xing, C. H. Sun, H. G. Yang and C. Z. Li, CrystEngComm, 2011, 13, 1378 RSC.
- Z.-H. Liu, K. Ooi, H. Kanoh, W.-P. Tang and T. Tomida, Langmuir, 2000, 16, 4154 CrossRef CAS.
- K. Kai, Y. Yoshida, H. Kageyama, G. Saito, T. Ishigaki, Y. Furukawa and J. Kawamata, J. Am. Chem. Soc., 2008, 130, 15938 CrossRef CAS.
- Y. Li, B. C. Zhang, X. W. Xie, J. L. Liu, Y. D. Xu and W. J. Shen, J. Catal., 2006, 238, 412 CrossRef CAS.
- N. G. Cho, I.-S. Hwang, H.-G. Kim, J.-H. Lee and I.-D. Kim, Sens. Actuators, B, 2011, 155, 366 CrossRef.
- C. W. Na, H. S. Woo and J. H. Lee, RSC Adv., 2012, 2, 414 RSC.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496 CrossRef CAS.
- R. C. Makkus, K. Hemmes and J. H. W. D. Wir, J. Electrochem. Soc., 1994, 141, 3429 CrossRef CAS.
- H. Kumagai, M. Matsumoto, K. Toyoda and M. Obara, J. Mater. Sci. Lett., 1996, 15, 1081 CrossRef CAS.
- S. C. Chae, J. S. Lee, S. Kim, S. B. Lee, S. H. Chang, C. Liu, B. Kahng, H. Shin, D. W. Kim, C. U. Jung, S. Seo, M. J. Lee and T. W. Noh, Adv. Mater., 2008, 20, 1154 CrossRef CAS.
- K. Oka, T. Yanagida, K. Nagashima, T. Kawai, J. S. Kim and B. H. Park, J. Am. Chem. Soc., 2010, 132, 6634 CrossRef CAS.
- C. Li, Y. Liu, L. Li, Z. Du, S. Xu, M. Zhang, X. Yin and T. Wang, Talanta, 2008, 77, 455 CrossRef CAS.
- X. Song and L. Gao, J. Phys. Chem. C, 2008, 112, 15299 CAS.
- B. Liu, H. Yang, H. Zhao, L. An, L. Zhang, R. Shi, L. Wang, L. Bao and Y. Chen, Sens. Actuators, B, 2011, 156, 251 CrossRef.
- Z. P. Wei, M. Arredondo, H. Y. Peng, Z. Zhang, D. L. Guo, G. Z. Xing, Y. F. Li, L. M. Wong, S. J. Wang, N. Valanoor and T. Wu, ACS Nano, 2010, 4, 4785 CrossRef CAS.
- H. Q. Cao, X. Q. Qiu, Y. Liang, L. Zhang, M. J. Zhao and Q. M. Zhu, ChemPhysChem, 2006, 7, 497 CrossRef CAS.
- L. Bai, F. Yuan, P. Hu, S. Yan, X. Wang and S. Li, Mater. Lett., 2007, 61, 1698 CrossRef CAS.
- W. Zhou, M. Yao, L. Guo, Y. M. Li, J. H. Li and S. H. Yang, J. Am. Chem. Soc., 2009, 131, 2959 CrossRef CAS.
- F. Jiao, A. H. Hill, A. Harrison, A. Berko, A. V. Chadwick and P. G. Bruce, J. Am. Chem. Soc., 2008, 130, 5262 CrossRef CAS.
- J. Hu, K. Zhu, L. F. Chen, H. J. Yang, Z. Li, A. Suchopar and R. Richards, Adv. Mater., 2008, 20, 267 CrossRef CAS.
- L. J. Guo, X. P. Shen, G. X. Zhu and K. M. Chen, Sens. Actuators, B, 2011, 155, 752 CrossRef.
- G. X. Zhu, Y. J. Liu, C. Zhang, Z. L. Zhu and Zheng Xu, Chem. Lett., 2010, 39, 994 CrossRef CAS.
- S. L. Wanga, L. Q. Qiana, H. Xua, G. L. Lüa, W. J. Donga and W. H. Tang, J. Alloys Compd., 2009, 476, 739 CrossRef.
- Q. Peng, Y. Dong and Y. D. Li, Angew. Chem., Int. Ed., 2003, 42, 3027 CrossRef CAS.
- W. Z. Wang, Y. K. Liu, C. K. Xu, C. L. Zheng and G. H. Wang, Chem. Phys. Lett., 2002, 362, 119 CrossRef CAS.
- N. Mironova-Ulmane, A. Kuzmin1, I. Steins, J. Grabis, I. Sildos and M. Pärs, J. Phys. Conf. Ser., 2007, 93, 012039 CrossRef.
- J. H. Lee, Sens. Actuators, B, 2009, 140, 319 CrossRef.
- J. Park, X. P. Shen and G. X. Wang, Sens. Actuators, B, 2009, 136, 494 CrossRef.
- T. He, D. Chen, X. Jiao, Y. Wang and Y. Duan, Chem. Mater., 2005, 17, 4023 CrossRef CAS.
- Z. J. Wang, Z. Y. Li, J. H. Sun, H. N. Zhang, W. Wang, W. Zheng and C. Wang, J. Phys. Chem. C, 2010, 114, 6100 CAS.
- S. B. Patil, P. P. Patil and M. A. More, Sens. Actuators, B, 2007, 125, 126 CrossRef.
- R.W. J. Scott, S. M. Yang, G. Chabanis, N. Coombs, D. E. Williams and G. A. Ozin, Adv. Mater., 2001, 13, 1468 CrossRef CAS.
- Q. Yu, C. Yu, W. Fu, M. Yuan, J. Guo, M. Li, S. Liu, G. Zou and H. Yang, J. Phys. Chem. C, 2009, 113, 12016 CAS.
- A. Tricoli, M. Righettoni and A. Teleki, Angew. Chem., Int. Ed., 2010, 49, 7632 CrossRef CAS.
- N. Barsan and U. Weimar, J. Electroceram., 2001, 7, 143 CrossRef CAS.
- G. Zhang and M. L. Liu, Sens. Actuators, B, 2000, 69, 144 CrossRef.
- F. Hernandez-Ramirez and J. D. Prades, Adv. Funct. Mater., 2008, 18, 2990 CrossRef CAS.
- P. Feng, X. Y. Xue, Y. G. Liu and T. H. Wang, Appl. Phys. Lett., 2006, 89, 243514 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Additional HRTEM images of hierarchical NiO microspheres, SEM, TEM, HRTEM images, plots of log(S − 1) vs. log(c), and XRD pattern of NiO nanoparticles. See DOI: 10.1039/c2ra01307j |
| This journal is © The Royal Society of Chemistry 2012 |
