Visible light-driven degradation of tetrabromobisphenol A over heterostructured Ag/Bi5Nb3O15 materials†
Yingna
Guo
a,
Ling
Chen
a,
Xia
Yang
b,
Fengyan
Ma
a,
Shengqu
Zhang
a,
Yuxin
Yang
b,
Yihang
Guo
*a and
Xing
Yuan
*b
aFaculty of Chemistry, Northeast Normal University, Changchun 130024, P.R. China. E-mail: guoyh@nenu.edu.cn (Y. Guo); Tel: +86 431 85098705
bCollege of Urban and Environmental Sciences, Northeast Normal University, Changchun 130024, P.R. China. E-mail: yuanx@nenu.edu.cn (X. Yuan); Tel: +86 431 85099561
First published on 2nd March 2012
Abstract
A mild wet chemical synthesis route, hydrothermal treatment–photodeposition, was developed to obtain a series of heterostructured metallic silver/bismuth niobate (Ag/Bi5Nb3O15) hybrid materials with a single-crystalline orthorhombic layered structure and photoresponse in both the UV and visible light region. As a novel alternative photocatalyst to TiO2, the photocatalytic activity of as-prepared Ag/Bi5Nb3O15 was evaluated by the degradation of an aqueous tetrabromobisphenol A (TBBPA, a member of thebrominated flame retardant family) under visible light irradiation (400 nm < λ < 680 nm and 420 nm < λ < 680 nm). Additionally, the influence of initial pH in the reaction system on TBBPA degradation was investigated. The excellent photocatalytic activity of the Ag/Bi5Nb3O15 materials was explained, and the degradation pathway of an aqueous TBBPA catalyzed by the Ag/Bi5Nb3O15 under the visible light irradiation was proposed.
1 Introduction
Photocatalytic oxidation of organic compounds based on TiO2 has been intensively studied and found to be successful in achieving the mineralization of various organic pollutants in water and air, especially for bioresistant organics.1 Nevertheless, the practical application of TiO2 to the degradation of organic compounds has been limited due to: (1) quantum yield of TiO2 is low owing to a fast recombination between the photoexcited holes (hVB+) and electrons (eCB−); and (2) TiO2 has no photoresponse under visible light irradiation, which severely limits the development of solar photocatalysis for environmental applications.2 Consequently, increasing research endeavours have been devoted to the search for novel photocatalytic materials that may overcome the limitations of TiO2. On the one hand, new TiO2-based photocatalytic materials were designed including metal or non-metal doping to modify the electronic structure of TiO23–7 and heterostructured design of integrated multi-semiconductor systems to promote the separation of hVB+ − eCB− pairs and/or to increase the sensitivity to sunlight of TiO2.2,8,9 On the other hand, alternative photocatalysts to TiO2 for solar applications have emerged during the last few years.10 Among these, metal oxides with d10 main group elements such as Bi(III),11–15 In(III),16,17 or Ga(III) 10,17 or mixed oxide semiconductors based on transition metals with d0 configurations such as Ta(V),12 Nb(V),13,16 Ti(IV),14 or V(V)9 are the most successful alternative photocatalysts. The representative systems include ABn-1NbnO3n+1 (A = K, Rb, Cs; B = Ca, Sr, Na, Pb; n = 3–4), InTaO4, InVO4, BiVO4, SrTiO3, Bi2WO6, Bi2MoO6, CaIn2O4, BiPO4, and BiOCl,10,18 and their photocatalytic performances were mainly studied by the degradation of various dye pollutants such as Rhodamine B, methylene blue, and methyl orange under both UV and visible light irradiation. Nevertheless, further assessment of their activities with light insensitive target compounds is necessary to exclude the degradation of organic pollutants assisted by the dye sensitization effect.Our recent work focuses on the development of a heterostructured metallic silver-layered bismuth niobate (Ag/Bi5Nb3O15) system as a novel and efficient alternative photocatalyst of TiO2 towards the degradation of a light insensitive compound. Bismuth salt photocatalysts, usually BiVO4, BiPO4, and Bi2WO6, have a narrow band gap due to the elevated valence band (VB) caused by the hybridization of the O2p orbital and the Bi6s orbital, and therefore, these bismuth salt photocatalysts exhibit excellent photocatalytic activities for the degradation of aqueous organic pollutants.19 Bi5Nb3O15 possess a mixed layered Aurivillius phase structure that can be expressed as [Bi2O2] + [NbO4] + [Bi2O2] + [BiNb2O4],20,21 and its photocatalytic efficiency is expected to be high enough since it is composed of both d10 main group elements and d0 transition metals. Additionally, deposition of silver particles in the interlayer space of the Bi5Nb3O15 compound is expected to give a positive influence on the photocatalytic activity of Bi5Nb3O15. On the one hand, silver is a noble metal that can act as an electron trapper to facilitate the separation of the hVB+ − eCB− pairs generated by light exciting Bi5Nb3O15 and thereby enhances the quantum efficiency of Bi5Nb3O15. On the other hand, it is well known that noble metals like gold, platinum, and silver exhibit a characteristic surface plasmon resonance (SPR) band in the visible-light region, and they also possess a considerable UV light response owing to the interband transition.22 Accordingly, deposition of Ag particles in the interlayer space of the Bi5Nb3O15 compound may harvest the full solar energy more efficiently. Consequently, the cooperation between metallic Ag and Bi5Nb3O15 is expected to lead to a Ag/Bi5Nb3O15 material with enhanced photocatalytic efficiency for the degradation of organic pollutants.
Bi5Nb3O15 was conventionally synthesized by a high temperature solid-state reaction, which results in the compound with large agglomerated particles, irregular morphology as well as a small BET surface area.21 Moreover, the photocatalytic performance of Bi5Nb3O15 has seldom been studied except for its ferroelectric and optoelectric properties. In our recently published communication, we reported a single-crystalline orthorhombic Ag/Bi5Nb3O15 heterostructure that was prepared by a mild hydrothermal method (200 °C) combined with photodeposition. The material exhibited excellent simulated sunlight (320 nm < λ < 680 nm) photocatalytic activity towards the degradation of a light insensitive compound, tetrabromobisphenol A (TBBPA), and its activity outperformed Bi5Nb3O15 itself and Degussa P25 TiO2.11 From the viewpoint of application of visible light photocatalyisis for pollution control, we herein further evaluate the visible light photocatalytic performance of the Ag/Bi5Nb3O15 (Ag loadings from 1% to 20%) towards TBBPA degradation in the regions of 400 nm < λ < 680 nm and 420 nm < λ < 680 nm. Meanwhile, in order to better understand Ag/Bi5Nb3O15-driven visible light photocatalysis, the degradation pathway of an aqueous TBBPA catalyzed by Ag/Bi5Nb3O15 under visible light irradiation is studied based on the identified intermediate products; additionally, photoelectrochemical experiments are conducted to confirm the enhanced quantum efficiency of the Ag/Bi5Nb3O15 with respect to Bi5Nb3O15. Finally, the influence of initial pH in the reaction system of TBBPA degradation is investigated.
For most of the heterogeneous photocatalysis studies, dyes have been extensively chosen as model pollutants. However, dyes exhibit a response in the visible light region, which leads to a dye sensitization effect and thereby complicates the real photocatalytic activity under visible light irradiation. Selecting TBBPA can avoid the case mentioned above; meanwhile, TBBPA is the most widely used brominated flame retardant in the treatment of paper, textiles, plastics, electronic equipment and upholstered furniture. TBBPA is a nonvolatile and a non-mobile agent in soils and river sediments because of its limited water solubility, however, it is expected to leach out into ground waters and rivers at higher acidity. More recently, concerns have arisen due to the increasing occurrence of TBBPA in the environment and to the proven toxic and endocrine disrupting activity. TBBPA is difficult to degrade under natural conditions, but it can be decomposed through anaerobic–aerobic biological process when microorganisms are domesticated for a long period.23 Therefore, how to efficiently treat the wastewaters containing TBBPA is an important issue in the protection of ecosystems.
2 Experimental
2.1 Catalyst preparation
The procedure follows our previous method.11 Bi(NO3)3·5H2O (2 mmol) and NbCl5 (1.4 mmol) was dissolved with ethanol (10 mL) under vigorous stirring for 0.5 h, respectively. With continuous stirring, the above NbCl5 solution was slowly added to the Bi(NO3)3 solution. NH3·H2O (13 mol L−1) was added into the mixture to adjust the acidity of the system to pH 9, and then the solution was magnetically stirred for 0.5 h to obtain a white suspension at room temperature. The white suspension was transferred into a Teflon-lined stainless steel autoclave, and the autoclave was heated to 473 K for 24 h. After reaction, the obtained Bi5Nb3O15 was filtered and washed with distilled water and ethanol three times, and then Bi5Nb3O15 was dried at 318 K for 12 h.For silver photodeposition, 625 mg of Bi5Nb3O15 was placed into 90 mL of aqueous AgNO3 solution with the concentration of 0.1 mg mL−1, 0.2 mg mL−1, 0.53 mg mL−1, 1.11 mg mL−1, and 2.51 mg mL−1, respectively, to obtain different Ag loadings. The photodeposition was carried out under UV-light irradiation provided by a 100 W high pressure mercury lamp for 2 h. The gray–black product was rinsed continuously with distilled water until no Ag+ ion was detected in the eluate, and finally it was dried for 12 h at 318 K. The product was denoted as Ag/Bi5Nb3O15-x, where x represents the Ag doping level (wt %) in the product.
2.2 Catalyst characterization
X-Ray diffraction (XRD) patterns were obtained with a Japan Rigaku D/max 2000 X-ray diffractometer. X-Ray photoelectron spectroscopy (XPS) was performed on a VG-ADES 400 instrument with Mg K-ADES source at a residual gas pressure of below 10−8 Pa. Transmission electron microscope (TEM), high resolution transmission electron microscope (HRTEM), and selected area electron diffraction (SAED) micrographs were recorded on a JEM-2100F high resolution transmission electron microscope at an accelerating voltage of 200 kV. Nitrogen porosimetry measurements were performed on a Micromeritics ASAP 2020 M surface area and porosity analyzer after the samples were outgassed under vacuum at 363 K for 1 h and 493 K for 12 h. UV-visible diffuse reflectance spectra (UV-vis/DRS) were recorded on a Cary 500 UV-vis-NIR spectrometer.2.3 Photocatalytic evaluation
The photocatalytic degradation of TBBPA was conducted in a quartz photoreactor (Scheme S1 of ESI†). The light source was provided by a PLS-SXE300 Xe lamp (300 W, Beijing Trusttech Co. Ltd., China), and the light intensity was adjusted to 150 mW cm−2 (1.5 AM) measured by a radiometer (OPHIR, Newport, USA). The IR irradiation from the Xe lamp was obviously weakened by an IR cut filter (λ > 680 nm, see Scheme S2 of ESI†), the UV-vis light irradiation (320 nm < λ < 680 nm) was provided by this lamp without adding any cut filter, and the visible light irradiation (400 nm < λ < 680 nm and 420 nm < λ < 680 nm) can be obtained by removing the UV irradiation from the lamp by using a 400 nm and 420 nm cut filter, respectively. For all adsorption and photocatalytic tests, 100 mL of TBBPA solution (initial concentration of 40 mg L−1, mixed water/acetonitrile solvent) and 150 mg of catalyst were used. After the adsorption–desorption equilibrium was established, the Xe lamp was turned on while the stir was applied continuously throughout the irradiation. The acidity of the suspension was neutral, and the temperature of the suspension was maintained at 303 ± 2 K by circulation of water through an external cooling jacket, and the system was open to air. Changes of TBBPA concentrations during photocatalytic process were analyzed by a Agilent 1200 high pressure liquid chromatography (HPLC). Intermediates produced during the TBBPA degradation were identified by a Applied Biosystem liquid chromatography (C8 column)-Q-Trap triple quadrupole mass spectrometer equipped with an electrospray ionization source (ESI-MS) and AutoflexIII smartbeam matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI-TOF/TOF/MALDI-TOF-MS, Bruker Daltonics Inc., Germany) in reflector negative mode with an accelerating voltage of 200 kV.2.4 Photoelectrochemical experiment
Photocurrent measurement was carried out using the conventional three electrode setup connected to an electrochemical station (CH Instrument 660C, Shanghai Chenhua, China). In this electrochemical system, Bi5Nb3O15/Ti or Ag/Bi5Nb3O15-10/Ti (effective area is 4 cm2) was used as a working photoanode, and a Pt sheet (size 30 × 40 mm, purity 99.99%) and an Ag/AgCl (saturated KCl) electrode were used as the counter electrode and reference electrode, respectively. The electrolyte was 0.01 mol L−1 Na2SO4 aqueous solution (110 mL), and the distance between Ti sheet and Pt sheet was fixed at 30 mm. A PLS-SXE300 Xe lamp was used to provide light irradiation. The distance between Ti sheet and light source was fixed at 80 mm. The measurements were carried out at a constant potential of +1.0 V to the working photoanode.3. Results and discussion
3.1 Characterization of Ag/Bi5Nb3O15
XRD analysis displayed in Fig. 1a shows that all of the diffraction peaks of the Bi5Nb3O15 are indexed to the orthorhombic phase (JCPDS 16-0293). As for the heterostructured Ag/Bi5Nb3O15 with Ag loading levels of 1%, 2%, 5%, 10%, and 20%, their main diffraction peaks are still in accordance with orthorhombic Bi5Nb3O15; meanwhile, the cubic Ag phase is also observed with the characteristic 2θ value of 38.1° (111) (JPCDS 87-0720). The chemical states of various elements in the Ag/Bi5Nb3O15 materials were examined through analyzing its high-resolution XPS spectra in the Ag3d, Bi4f, Nb3d, and O1s binding energy region (Fig. S1 of ESI†). The results show that silver in the Ag/Bi5Nb3O15 is of metallic nature24 with Ag3d5/2 and Ag3d3/2 binding energies of 368.2 eV and 374.2 eV and a spin–orbit energy separation of 6.0 eV (Fig. S1a of ESI†). In the case of Bi, it is mainly in the Bi3+ species with a binding energy of 164.2 eV and 158.9 eV and a spin–orbit splitting of 5.3 eV (Fig. S1b of ESI†).25 The shoulder peak at 162.4 eV evidences a lower Bi oxidation state (Bi2+).26 As for Nb in Ag/Bi5Nb3O15, it exists in the Nb5+ form with a characteristic binding energy of 209.5 eV and 206.6 eV (Fig. S1c of ESI†).27 Finally, the O1s XPS spectrum shows three unseparated peaks, which are assigned to different oxygen species such as lattice oxygen (530.0 eV), hydroxyl oxygen (531.6 eV), and adsorbed oxygen (533.1 eV) at the surface of Ag/Bi5Nb3O15 (Fig. S1d of ESI†).28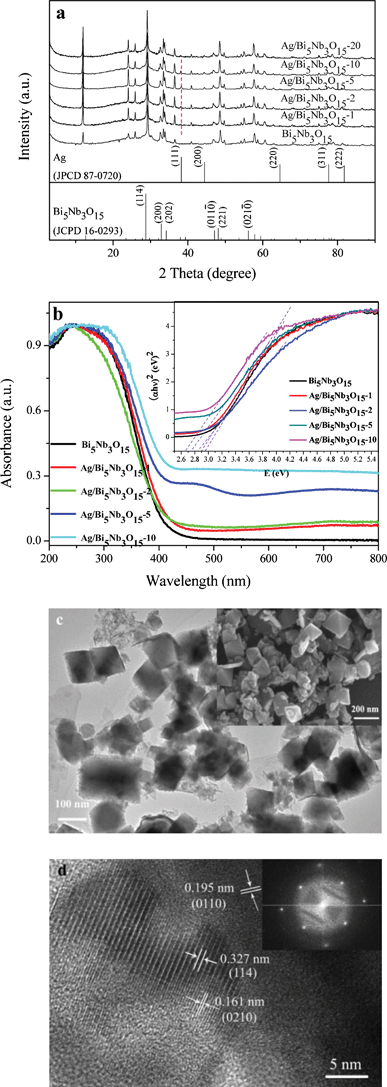 | ||
| Fig. 1 (a) XRD patterns of Ag/Bi5Nb3O15 materials; (b) UV-vis/DRS of Bi5Nb3O15 and Ag/Bi5Nb3O15 materials. Inset: plots of (αhν)2vs. hν for Bi5Nb3O15 and Ag/Bi5Nb3O15 materials; (c) TEM image of Ag/Bi5Nb3O15-10 material. Inset: SEM image of Ag/Bi5Nb3O15-10 material; and (d) HRTEM image of Ag/Bi5Nb3O15-10 material. Inset: SAED pattern of Ag/Bi5Nb3O15-10 material. | ||
UV-vis/DRS analysis displayed in Fig. 1b indicates that pure Bi5Nb3O15 shows a light response in the range of 200 nm to 450 nm with a steep edge, attributing to a bandgap transition from the valence band (the hybridization of O2p orbital and Bi6s orbital) to the conduction band (Nb4d orbital) of the compound.29 After deposition of silver particles, the bandgap band of Bi5Nb3O15 has some redshift accompanied by a new absorption band in the visible light region (400 nm–800 nm). This new absorption is typical of the silver SPR band, further substantiating the formation of Ag0 in as-prepared Ag/Bi5Nb3O15 materials.30 The above result indicates that Ag/Bi5Nb3O15 materials possess full spectrum absorption across both the UV and visible light region. This optical absorption property is expected to find important applications in photocatalysis since more visible light energy might be effectively harvested by the combination of the charge transfer of Bi5Nb3O15 and the SPR effect of metallic Ag. Based on the Kubelka–Munk formula αhν = A (hν − Eg)n/2, where α, ν, Eg and A are the absorption coefficient, the light frequency, the band gap and a constant, respectively.31 The bandgap (Eg) of Bi5Nb3O15 and Ag/Bi5Nb3O15-1, Ag/Bi5Nb3O15-2, Ag/Bi5Nb3O15-5, Ag/Bi5Nb3O15-10 is therefore estimated to be ca. 2.92 eV, 2.90 eV, 2.85 eV, 2.75 eV, and 2.65 eV from the onset of the absorption edges (inset of Fig. 1b).
The representative TEM and FESEM images of Ag/Bi5Nb3O15 reveal that the material mainly possesses an octahedral shape with a particle size of 50 nm–200 nm (Fig. 1c). However, Ag particles are hardly observed from the above two images. HRTEM observation further confirms the orthorhombic phase of the Bi5Nb3O15 with characteristic lattice fringes of 0.327 nm (114), 0.195 nm (011![[0 with combining macron]](https://www.rsc.org/images/entities/char_0030_0304.gif) ), and 0.161 nm (021
), and 0.161 nm (021![[0 with combining macron]](https://www.rsc.org/images/entities/char_0030_0304.gif) ), respectively (Fig. 1d). The SAED pattern indicates that the Ag/Bi5Nb3O15 exhibits a single-crystalline orthorhombic phase (inset of Fig. 1d).
), respectively (Fig. 1d). The SAED pattern indicates that the Ag/Bi5Nb3O15 exhibits a single-crystalline orthorhombic phase (inset of Fig. 1d).
Both Bi5Nb3O15 and Ag/Bi5Nb3O15 show type IV nitrogen adsorption–desorption isotherms with a H3 hysteresis loop, characteristics of the mesoporous materials are formed due to the aggregation of the primary nanocrystallites (Fig. S2 of ESI†).9 The determined BET surface areas (from 44 m2 g−1 to 29 m2 g−1) of the Ag/Bi5Nb3O15 materials decrease gradually as Ag loading is increased from 0% to 20%, implying that the part of the interlayer space of Bi5Nb3O15 was occupied by Ag particles.
3.2 Photocatalytic property of Ag/Bi5Nb3O15
The photocatalytic property of the as-prepared Ag/Bi5Nb3O15 was evaluated by the degradation of a light insensitive compound, TBBPA (initial concentration 40 mg L−1), in a water–CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solution under visible light irradiation. As a comparison, pure Bi5Nb3O15 and Degussa P25 TiO2 systems were also tested under the same conditions; in addition, the activity of the Ag/Bi5Nb3O15 under simulated sunlight (full solar spectrum) irradiation was also tested.
1) mixed solution under visible light irradiation. As a comparison, pure Bi5Nb3O15 and Degussa P25 TiO2 systems were also tested under the same conditions; in addition, the activity of the Ag/Bi5Nb3O15 under simulated sunlight (full solar spectrum) irradiation was also tested.
Fig. 2a shows that a good dispersion and adsorption–desorption equilibrium between TBBPA molecules and the catalyst surface was reached after stirring the suspension of an aqueous TBBPA and the catalyst in the dark for 30 min, and the Ag/Bi5Nb3O15 material with the highest Ag loading (20%) possessed the highest adsorption capacity for TBBPA molecules. Direct photolysis experiments indicate that changes of the concentrations of TBBPA in the absence of any catalyst are negligible under the full solar spectrum irradiation (320 nm < λ < 680 nm) for 120 min.
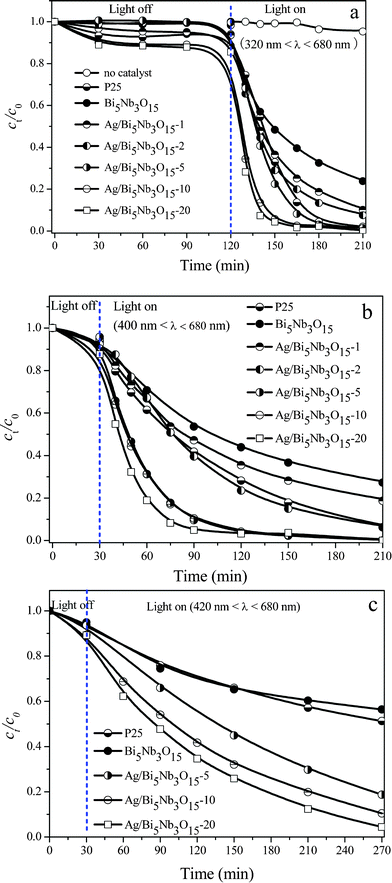 | ||
| Fig. 2 Photocatalytic activity of pure Bi5Nb3O15, Ag/Bi5Nb3O15, and Degussa P25 towards TBBPA degradation. (a) Influence of Ag loadings under the irradiation at 320 nm < λ < 680 nm; (b) irradiation at 400 nm < λ < 680 nm; and (c) irradiation at 420 nm < λ < 680 nm. Initial concentration of TBBPA 40 mg L−1, volume 100 mL, catalyst amount 150 mg. | ||
Fig. 2a also gives the interesting results concerning the photocatalytic activity of pure Bi5Nb3O15, Ag/Bi5Nb3O15, and Degussa P25 TiO2 towards TBBPA degradation under the simulated sunlight (320 nm < λ < 680 nm) irradiation without equipping any UV cut filter. Under these conditions, pure Bi5Nb3O15 exhibits the lowest photocatalytic activity; however, enhanced photocatalytic activity is obtained after deposition of Ag on the Bi5Nb3O15; moreover, the photocatalytic activity of the Ag/Bi5Nb3O15 materials increases gradually with the Ag loading. For example, conversion of TBBPA reached 52.6%, 63.5%, 71.0%, 77.5%, 94.3%, and 95.7%, respectively, after irradiating the Bi5Nb3O15, Ag/Bi5Nb3O15-1, Ag/Bi5Nb3O15-2, Ag/Bi5Nb3O15-5, Ag/Bi5Nb3O15-10, and Ag/Bi5Nb3O15-20 for 30 min. Importantly, the Ag/Bi5Nb3O15 materials with Ag loading equal to or higher than 5% showed higher photocatalytic activity than that of Degussa P25 TiO2.
Fig. 2b presents the photocatalytic activity of pure Bi5Nb3O15, Ag/Bi5Nb3O15, and Degussa P25 TiO2 towards the degradation of TBBPA under visible light (400 nm < λ < 680 nm) irradiation. The result shows that all tested photocatalysts follow a similar activity order under visible light irradiation with respect to that tested under UV light irradiation. Nevertheless, the degradation rate of TBBPA under the visible light irradiation is somewhat slower: for the most photoactive Ag/Bi5Nb3O15-20, conversion of TBBPA reached 81.1% under the light with 400 nm < λ < 680 nm irradiation for 30 min; as for Degussa P25 TiO2, conversion of TBBPA reached 38.5% under the same conditions.
Ag/Bi5Nb3O15 still exhibited excellent photocatalytic activity under the light of 420 nm < λ < 680 nm irradiation although the degradation rate of TBBPA became slower (Fig. 2c). In this circumstance, Ag/Bi5Nb3O15-20 and Ag/Bi5Nb3O15-10 are still more photoactive. For example, conversion of TBBPA reached 31.1% after the light irradiation of Ag/Bi5Nb3O15-10 for 30 min, and further increased the light irradiation time to 240 min, conversion of TBBPA approached 90%. As for the Degussa P25 TiO2 and pure Bi5Nb3O15, conversion of TBBPA approaches 45% after the light irradiation for 240 min.
Finally, influence of initial pH in the reaction system on TBBPA degradation was investigated in the range from pH 3 to 12 by selecting Ag/Bi5Nb3O15-10 as the representative photocatalyst under the simulated sunlight (320 nm < λ < 680 nm) irradiation. From the results shown in Fig. 3 it is found that the adsorption capacity of the Ag/Bi5Nb3O15-10 for TBBPA molecules is similar at various initial pH values. Additionally, the acidity of the reaction system influences the photocatalytic activity of the Ag/Bi5Nb3O15-10 significantly. The Ag/Bi5Nb3O15-10 showed the highest photocatalytic activity at nearly neutral conditions (pH 5–7), and this activity decreased considerably once the degradation reaction was performed under acidic (pH 3) or alkaline (pH 9–12) conditions. Under the acidic conditions, abundant H+ can inhibit the efficient generation of hydroxyl radicals on the catalyst surface, and thereby decreasing the reaction rate. It is generally accepted that the photocatalytic activity is higher when the reaction is conducted under alkaline conditions since an increase of OH− ion concentration can facilitate the generation of hydroxyl radicals in the reaction system.32,33 However, in the Ag/Bi5Nb3O15-catalyzed TBBPA degradation system, lower activity under alkaline conditions can be attributed to the special layered structure of Bi5Nb3O15. It has been reported that the structure distortion can facilitate the separation of hVB+ − eCB− pairs obviously.34 However, the distortion effect of [Bi2O2]2+ layers to NbO6 octahedral can be weakened once the plentiful OH− ion exists in the interlayer space of Bi5Nb3O15. This leads to the decreased separation efficiency of hVB+ − eCB− pairs and thereby a lower photacatalytic activity.
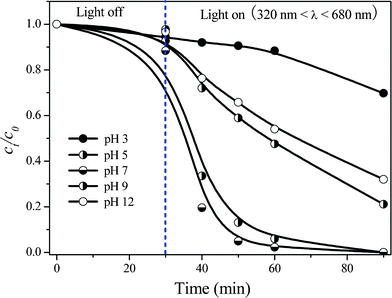 | ||
| Fig. 3 Influence of initial pH on the degradation of aqueous TBBPA in Ag/Bi5Nb3O15-10/simulated sunlight system (320 nm < λ < 680 nm). Initial concentration of TBBPA 40 mg L−1, volume 100 mL, catalyst amount 150 mg. | ||
3.3 Photocatalytic mechanism
The above excellent photocatalytic activity of the Ag/Bi5Nb3O15 towards TBBPA degradation originates from the intrinsic structural characteristics of the single-crystalline orthorhombic layered Bi5Nb3O15 itself. That is, the active sites that exist in the layered photocatalyst only need to diffuse a very short distance to contact with the reactants; at the same time, the introduction of metallic Ag particles throughout the interlayer of the Bi5Nb3O15 also gives an important contribution to the photocatalytic activity of the Bi5Nb3O15 materials. Under the light with suitable energy irradiation of the Ag/Bi5Nb3O15 hybrid photocatalyst, Bi5Nb3O15 is excited and the eCB− and hVB+ are generated at the surface and/or interlayer space of the Bi5Nb3O15 compound (eqn (1)). Owing to the standard redox potential of BiV/BiIII (E0 = + 1.59 eV at pH 0) is more negative than that of OH•/OH− (+ 1.99 eV), the hVB+ cannot react with OH−/H2O to form OH• radicals directly. However, O2•− radicals can be formed based on eqn (2). Meanwhile, OH• radicals are generated indirectly followed by eqn (3)–(4). The above three kinds of active species (hVB+, OH•, and O2•−) are responsible for the photocatalytic oxidation of TBBPA to produce the final products including Br− ion, H2O, and CO2 with the aid of continuous light irradiation (eqn (5)). | (1) |
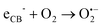 | (2) |
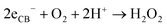 | (3) |
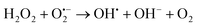 | (4) |
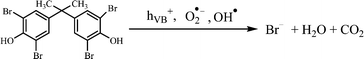 | (5) |
On the other hand, because of lower Fermi level of metallic Ag, the eCB− at the surface or interlayer space of Bi5Nb3O15 can be trapped by Ag particles.35 Therefore, a more effective separation of hVB+ − eCB− pairs and interfacial charge transfer occurred in the Ag/Bi5Nb3O15-catalyzed TBBPA degradation system in comparison to that of the pure Bi5Nb3O15 system. This enhanced quantum efficiency was investigated by the photoelectrochemical experiments in the Xe lamp irradiated Bi5Nb3O15/Ti and Ag/Bi5Nb3O15/Ti working electrode systems. Herein, the above working electrodes were obtained by spin-coating the Bi5Nb3O15-ethanol or Ag/Bi5Nb3O15-ethanol suspension on the Ti sheet. From photocurrent–time (I–t) profiles shown in Fig. 4 it can be seen that the sharp increased photocurrent responses appeared for Bi5Nb3O15/Ti and Ag/Bi5Nb3O15-10/Ti electrodes once the pulse light irradiation was applied, and that the Ag/Bi5Nb3O15-10/Ti electrode showed a higher photocurrent response than that of the Bi5Nb3O15/Ti electrode under the same testing conditions. The prompt increase in photocurrent response from light-off to light-on state is mainly ascribed to the fast transfer of the photogenerated eCB− on the surface of the Bi5Nb3O15/Ti-based electrodes. Accordingly, it is inferred that the transfer of eCB− at the surface of the Ag/Bi5Nb3O15/Ti electrode is faster than that of the Bi5Nb3O15/Ti electrode.
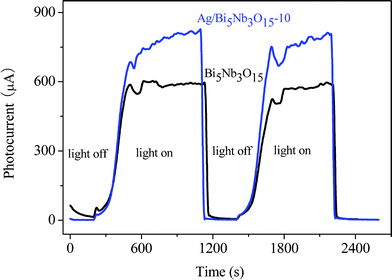 | ||
| Fig. 4 Photocurrent responses of Bi5Nb3O15/Ti and Ag/Bi5Nb3O15-10/Ti electrodes in 0.01 mol L−1 Na2SO4 electrolyte solution under UV illumination (the working electrode potential was constant at + 1.0 V). | ||
In addition, Ag particles can also been excited under the same conditions, which leads to an increased population of hVB+ and eCB− and thereby more active species in the current photocatalytic system with respect to the Ag-free Bi5Nb3O15 system.35–37 Consequently, it is reasonably inferred that Ag can have an additional role as a light harvester besides electron trapper in the current photocatalytic system, which is supported by the fact that the simulated sunlight photocatalytic activity of the Ag/Bi5Nb3O15 materials increased considerably with Ag loadings from 1% to 20%. The above discussion is consistent with our previously proposed band structure of Ag/Bi5Nb3O15 as well as the TBBPA degradation process over the simulated sunlight irradiated Ag/Bi5Nb3O15.11
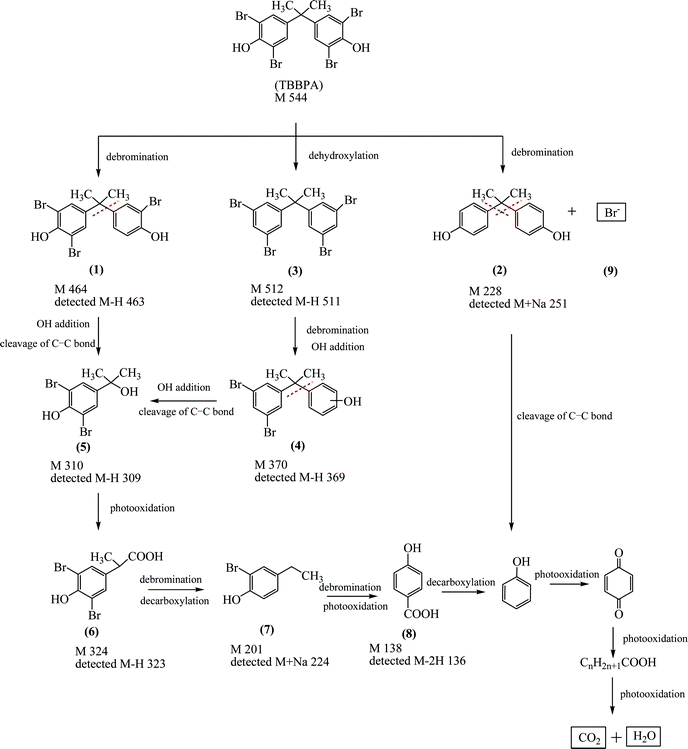 | ||
| Scheme 1 Proposed degradation pathway of aqueous TBBPA in the Ag/Bi5Nb3O15-10/visible light system. | ||
LC/ESI-MS, MALDI-TOF/TOF-MS, together with IC (Fig. S3 of ESI†) identified 9 intermediate products (Compounds 1–9 in Scheme 1) yielded during the course of the degradation of an aqueous TBBPA catalyzed by the Ag/Bi5Nb3O15 with the assistance of visible light (400 nm < λ < 680 nm). Based on the intermediates identified and the related literature work, the possible degradation pathway of an aqueous TBBPA in the current photocatalytic system is proposed and presented in Scheme 1. It shows that total degradation of TBBPA suffered from the steps of debromination, dehydroxylation, OH group addition, cleavage of the C–C bond, and decarboxylation. Finally, inorganic products including Br− ions, CO2, and H2O were yielded. At first, debromination occurred due to the cleavage of one and four C–Br bond(s) in a TBBPA molecule under the attack of the above active species, which leads to tribromobisphenol A (Compound 1) and bisphenol A (Compound 2) accompanied by the release of Br− ions (Compound 9); additionally, TBBPA may suffer from dehydroxylation to produce 2,2-bis(3,5-dibromophenyl)propane (Compound 3). Secondly, debromination and OH group addition from Compound 3 resulted in 2-(3,5-dibromophenyl)propan-2-yl-phenol (Compound 4). Subsequently, 4-(2-hydroxyisopropyl)-2,6-dibromophenol (Compound 5) was formed after the cleavage of the C–C bond from compound 1 or 4. 2-(3,5-dibromo-4-hydroxyphenyl)propanoic acid (Compound 6) can be easily formed after photooxidation of Compound 5. Simultaneous decarboxylation and debromination of Compound 6 led to 2-bromo-4-ethylphenol (Compound 7). Further debromination and photooxidation of Compound 7 led to 4-hydroxybenzoic acid (Compound 8). Decarboxylation of Compound 8 or the cleavage of the C–C bond from bisphenol A (Compound 2) yielded phenol and then quinine. Finally, a ring-opening reaction occurred, resulting in a series of aliphatic acids with the length of carbon lower than 6. Further increasing the simulated sunlight irradiation time resulted in the final products, CO2 and H2O.
Conclusions
At suitable Ag loadings (5%–20%), as-prepared Ag/Bi5Nb3O15 with a single-crystalline orthorhombic layered structure exhibited much higher visible light photocatalytic activity towards the degradation of a kind of brominated flame retardant, TBBPA, in comparison to Bi5Nb3O15 itself and Degussa P25 TiO2. By analyzing the identified intermediate products and photoelectrochemical test results it is inferred that this excellent photocatalytic activity originates from the intrinsic structure characteristic of Bi5Nb3O15; meanwhile, the introduction of metallic silver particles throughout the interlayer of the Bi5Nb3O15 also gives an important contribution due to facilitating the separation of the photoexcited hole-electron pairs as well as light harvesting ability in both the UV and visible light regions. The research reveals that the as-prepared Ag/Bi5Nb3O15 exhibits potential applications for the total decomposition of brominated flame retardants with the help of visible light.Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (09QNTD004), the Natural Science Fund Council of China (21173036 and 51008056), and the Science and Technology Project of Jilin Province (201101002).References
- U. Gaya and A. Abdullah, J. Photochem. Photobiol., C, 2008, 9, 1 CrossRef CAS.
- G. Liu, L. Wang, H. Yang, H. Cheng and G. Lu, J. Mater. Chem., 2010, 20, 831 RSC.
- K. Li, X. Yang, Y. Guo, F. Ma, H. Li, L. Chen and Y. Guo, Appl. Catal., B, 2010, 99, 364 CrossRef CAS.
- H. Li, Z. Bian, J. Zhu, Y. Huo, H. Li and Y. Lu, J. Am. Chem. Soc., 2007, 129, 4538 CrossRef CAS.
- J. Yu, G. Li, X. Wang, X. Hu, C. Leung and Z. Zhang, Chem. Commun., 2006, 2717 RSC.
- D. Mitoraj and H. Kisch, Angew. Chem., Int. Ed., 2008, 47, 9975 CrossRef CAS.
- G. Wu, J. Wang, D. Thomas and A. Chen, Langmuir, 2008, 24, 3503 CrossRef CAS.
- X. Yang, Y. Wang, L. Xu, X. Yu and Y. Guo, J. Phys. Chem. C, 2008, 112, 11481 CAS.
- X. Yang, F. Ma, K. Li, Y. Guo, J. Hu, W. Li, M. Huo and Y. Guo, J. Hazard. Mater., 2010, 175, 429 CrossRef CAS.
- M. Hernández-Alonso, F. Fresno, S. Suárez and J. Coronado, Energy Environ. Sci., 2009, 2, 1231 Search PubMed.
- Y. Guo, L. Chen, F. Ma, S. Zhang, Y. Yang, X. Yuan and Y. Guo, J. Hazard. Mater., 2011, 189, 614 CrossRef CAS.
- G. Zhang, M. Li, S. Yu, S. Zhang, B. Huang and J. Yu, J. Colloid Interface Sci., 2010, 345, 467 CrossRef CAS.
- H. Kim, D. Hwang and J. Lee, J. Am. Chem. Soc., 2004, 126, 8912 CrossRef CAS.
- X. Zhu, J. Zhang and F. Chen, Chemosphere, 2010, 78, 1350 CrossRef CAS.
- Y. Guo, X. Yang, F. Ma, K. Li, L. Xu, X. Yuan and Y. Guo, Appl. Surf. Sci., 2010, 256, 2215 CrossRef CAS.
- L. Zhang, I. Djerdj, M. Cao, M. Antonietti and M. Niederberger, Adv. Mater., 2007, 19, 2083 CrossRef CAS.
- H. Dong, Z. Li, X. Xu, Z. Ding, L. Wu, X. Wang and X. Fu, Appl. Catal., B, 2009, 89, 551 CrossRef CAS.
- C. Pan and Y. Zhu, Environ. Sci. Technol., 2010, 44, 5570 CrossRef CAS.
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459 CrossRef CAS.
- G. Li, D. Zhang, J. Yu and M. Leung, Environ. Sci. Technol., 2010, 44, 4276 CrossRef CAS.
- S. Tahara, A. Shimada, N. Kumada and Y. Sugahara, J. Solid State Chem., 2007, 180, 2517 CrossRef CAS.
- X. Chen, Z. Zheng, X. Ke, E. Jaatinen, T. Xie, D. Wang, C. Guo, J. Zhao and H. Zhu, Green Chem., 2010, 12, 414 RSC.
- W. James, E. Donna, J. Kristi and M. Haggblom, Environ. Sci. Technol., 2002, 36, 696 CrossRef.
- E. Stathatos, P. Lianos, P. Falaras and A. Siokou, Langmuir, 2000, 16, 2398 CrossRef CAS.
- G. Zhang, J. Yang, S. Zhang, Q. Xiong, B. Huang, J. Wang and W. Gong, J. Hazard. Mater., 2009, 172, 986 CrossRef CAS.
- A. Gulino, S. Delfa, I. Fragala and R. Egdell, Chem. Mater., 1996, 8, 1287 CrossRef CAS.
- K. Tabata, T. Choso and Y. Nagasawa, Surf. Sci., 1998, 408, 137 CrossRef CAS.
- L. Zhang, D. Chen and X. Jiao, J. Phys. Chem. B, 2006, 110, 2668 CrossRef CAS.
- L. Zhou, W. Wang, L. Zhang, H. Xu and W. Zhu, J. Phys. Chem. C, 2007, 111, 13659 CAS.
- T. Hirakawa and P. Kamat, J. Am. Chem. Soc., 2005, 127, 3928 CrossRef CAS.
- L. Wang, W. Wang, M. Shang, S. Sun, W. Yin, J. Ren and J. Zhou, J. Mater. Chem., 2010, 20, 8405 RSC.
- R. Jain and M. Shrivastava, J. Hazard. Mater., 2008, 152, 216 CrossRef CAS.
- R. Wang, D. Ren, S. Xia, Y. Zhang and J. Zhao, J. Hazard. Mater., 2009, 169, 926 CrossRef CAS.
- X. Chen, S. Shen, L. Guo and S. Mao, Chem. Rev., 2010, 110, 6503 CrossRef CAS.
- F. Zhang, Y. Pi, J. Cui, Y. Yang, X. Zhang and N. Guan, J. Phys. Chem. C, 2007, 111, 3756 CAS.
- C. He, Y. Yu, X. Hu and A. Larbot, Appl. Surf. Sci., 2002, 200, 239 CrossRef CAS.
- B. Xin, Z. Ren, H. Hu, X. Zhang, C. Dong, K. Shi, L. Jing and H. Fu, Appl. Surf. Sci., 2005, 252, 2050 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra01278b/ |
| This journal is © The Royal Society of Chemistry 2012 |
