Surfactant-assisted hydrothermal synthesis of Bi2O3 nano/microstructures with tunable size
Hongbing
Lu
ab,
Shimin
Wang
a,
Li
Zhao
a,
Binghai
Dong
a,
Zuxun
Xu
a and
Jinchai
Li
*b
aMinistry-of-Education Key Laboratory for the Green Preparation and Application of Functional Materials, Faculty of Materials Science and Engineering, Hubei University, Wuhan 430062, China
bKey Laboratory of Artificial Micro- and Nano-structures of Ministry of Education and School of Physics and Technology, Wuhan University, Wuhan 430072, China. E-mail: jinchaili@163.com
First published on 27th February 2012
Abstract
A novel and simple citrate-assisted solution approach has been developed for the shape-selective synthesis of Bi2O3 nanostructures with controllable bandgaps and morphologies at a relatively low temperature of 40 °C. Different distinctive morphologies, including nanorods, nanoplates, plate-built cylinders, nanoplates with holes, and nanorings, are created due to the selective adsorption of the citrate molecules on certain faces during crystal growth. The bandgaps and aspect ratios of the Bi2O3 nanostructures are easily tuned by modifying the product morphologies by adjusting the amount of trisodium citrate. More novel and complex Bi2O3 nanostructures with controllable morphologies and sizes can be manufactured with our method by optimizing the experimental parameters. The distinctive nanostructures presented here extend the family of Bi2O3 nanostructures, and they also provide new opportunities for exploring the potential applications of Bi2O3 in a number of fields including photocatalysis, gas sensors, and photoelectrochemistry.
1. Introduction
The physical properties of inorganic nanostructures fundamentally relate to their chemical composition, size, crystal structure, orientation, aspect ratio, and shape distributions.1,2 Remarkably, some properties, such as luminescence, photocatalytic, and sensing properties, are strongly dependent on the particular exposed surface of the nanostructures.3,4 The ability to synthetically tune these material parameters provides the opportunity to study and understand the relationship between chemical, structural, and quantum effects that occur uniquely at the nanoscale.Bismuth oxide (Bi2O3) has attracted continuous attention over the last three decades. It has five polymorphic forms that are labeled as α-Bi2O3 (monoclinic), β-Bi2O3 (tetragonal), γ-Bi2O3 (body centred cubic), δ-Bi2O3 (cubic), and ω-Bi2O3 (triclinic).5 Among them, the low-temperature α-phase and the high-temperature δ-phases are stable, but the others are high-temperature metastable phases.6 Bi2O3 has been widely used in photocatalysis, gas sensors, capacitors, optical coatings, photovoltaic cells, and so on, owing to their peculiar properties, such as large bandgap, high refractive index and dielectric permittivity, as well as marked photoconductivity.7–12 For the aforementioned applications, polymorph, particle size, porosity, aspect ratio, and specific surface area are of major importance. Recently, polymorph-tuned synthesis of α- and β-Bi2O3 nanowires has been demonstrated by controlling the Bi precursor heating temperature and/or substrate temperature via a vapor transport method.13 In addition, Bi2O3 nano/microstructures have been synthesized in various interesting morphologies, such as nanowires,9,12,14–16 nanorods,17 nanobelts,18 nanotubes,19–21etc., using several different methods, i.e., thermal evaporation,14,15 metalorganic chemical vapor deposition (MOCVD),17,18 template-based heat-treatment,19,20 and so on. Among various nanomaterials, 2-D anisotropic nanomaterials are especially attractive due to their high surface-to-volume ratio and high proportion of exposed high-index planes, which usually exhibit much higher chemical activity because high-index planes have high densities of atom steps, ledges, kinks, and dangling bonds.22 In particular, recent developments on 2-D oxide nanosheets have sparked tremendous research interest for exploring the synthesis and physical properties of 2-D nanomaterials as they are potentially useful not only for developing a new generation of optoelectronic devices, but also for high performance catalysts.23,24 However, the previously reported synthetic methods are mainly limited to the formation of the one-dimensional (1-D) Bi2O3 nanostructures. The production of 2-D and other complex Bi2O3 nanostructures has lingered far behind. Only several papers on the synthesis of 2-D Bi2O3 nanostructures have been published. Zhao et al.25 obtained Bi2O3 nanosquaresheets with typical length and thickness in the range of 200–600 nm and 30–100 nm by thermal evaporation of commercial Bi2O3 powder at a high temperature of 950 °C. Yu et al.26 synthesized α-Bi2O3 nanoplates with a thickness of 30–90 nm via a hydrothermal recrystallization technique by using commercial α-Bi2O3 as the starting material. Very recently, Bhande et al.27 prepared β-Bi2O3 nanoplates with a thickness of 100–400 nm via a wet chemical route. It is therefore highly necessary to further explore simple and low-cost approaches to the morphologically controlled synthesis of 2-D Bi2O3 nano/microstructures and other nanostructures. Large-scale use will require the development of simple, low-cost techniques for the synthesis of inorganic functional nanomaterials. One such method is to grow Bi2O3 nanostructures from an aqueous solution at low temperatures. Here, we develop a citrate-assisted low-temperature solution method for the rational synthesis of Bi2O3 nanostructures with distinctive shapes, including nanorods, nanoplates, plate-built cylinders, nanoplates with holes, and nanorings. The characteristic bandgap of Bi2O3 varies with product morphology according to the absorption spectrum investigations.
2. Experimental
Preparation of samples
In a typical synthesis, Bi(NO3)3·6H2O and hexamethylenetetramine (HMT) of equal molar ratio were dissolved in 50 mL of distilled water to form a 0.005 M solution, followed by the addition of reagent grade trisodium citrate with different molar ratios of citrate ion/Bi3+ (0.02∼10). The cleaned glass and Si(100) p-type substrates were placed at the bottom of sealed bottles containing the above solution. Then, the bottles were put into a water tank and maintained at 40 °C for 3 h. Finally, the products on the substrates were washed with deionized water and ethanol several times.Characterization
The morphology and crystalline structure of the samples were characterized by a Sirion FEG scanning electron microscope (SEM) in conjunction with energy-dispersive X-ray spectroscopy (EDX), D8 advanced X-ray diffraction (XRD), JEOL JEM 2010 transmission electron microscopy (TEM), and high-resolution transmission electron microscopy (HRTEM). Optical absorption spectra were recorded using a UV-vis-NIR dual-beam spectrophotometer (Varian Cary5000) at wavelengths from 800 to 200 nm.3. Results and discussion
Fig. 1a shows a typical low-magnification SEM image of the sample synthesized with a molar ratio of citrate ion/Bi3+ of 1. Obviously, the as-synthesized product is dominated by hexagonal-based thin plates of uniform size and well-defined shape. The average thickness and diameter of these nanoplates are about 170 nm and 2 μm, respectively. An XRD pattern of these nanoplates was also recorded and is presented in Fig. 1b. According to the diffraction peak locations and their relative intensities, the sample can be indexed to body centred cubic γ-Bi2O3 structure (JCPDS Card No. 06-0312). To further investigate the product morphology and structure, TEM was employed. Fig. 1c shows the typical TEM image of a single Bi2O3 nanoplate. It is apparent that the nanoplate possesses a hexagonally symmetric shape. The corresponding selected area electron diffraction (SAED) pattern of the nanoplate is exhibited in the top-right of Fig. 1c. The SAED pattern can be identified as [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11] zone axis body centred cubic Bi2O3, revealing that the as-prepared nanoplate is a single-crystal structure. The corresponding high-resolution TEM (HRTEM) image of the nanoplate is further illustrated in the bottom-right of Fig. 1c. Here the six-fold rotational symmetry and the {110} lattice fringes in three directions can be clearly resolved. These results reveal that the top and bottom surfaces of the nanoplates are {
11] zone axis body centred cubic Bi2O3, revealing that the as-prepared nanoplate is a single-crystal structure. The corresponding high-resolution TEM (HRTEM) image of the nanoplate is further illustrated in the bottom-right of Fig. 1c. Here the six-fold rotational symmetry and the {110} lattice fringes in three directions can be clearly resolved. These results reveal that the top and bottom surfaces of the nanoplates are {![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11} planes, whereas the six side surfaces are {110} planes.
11} planes, whereas the six side surfaces are {110} planes.
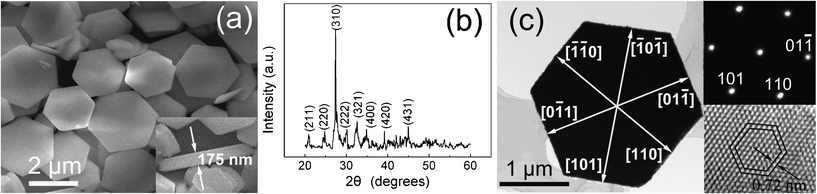 | ||
| Fig. 1 (a) Typical SEM image of the product synthesized with a molar ratio of citrate ion/Bi3+ of 1, exhibiting plate-like structures; (b) XRD pattern of the product; (c) TEM image of a single nanoplate with crystal orientations indicated; the top-right and bottom-right insets show the corresponding SAED pattern and HRTEM image of the nanoplate, respectively. | ||
Several experiments were carried out to explore the parameters that are important for the formation of nanoplates: (1) The reaction temperature has no obvious influence on the formation of nanoplates. Similar nanoplates can be produced at 90 °C or at even higher temperatures (e.g., 150 °C). (2) The solution concentration of Bi(NO3)3·6H2O and HMT is not crucial to the formation of shape-controlled nanoplates. Other stronger alkaline reagents, such as NH3·H2O and NaOH, also have no visible impact on the formation of nanoplates. (3) Controlling the experiments without trisodium citrate in the solution did not produce nanoplates but long nanorods instead (Fig. 2a). When a smaller quantity of trisodium citrate (citrate ion/Bi3+ of 0.02) was added, short nanorods with larger diameters were obtained (Fig. 2b). Further investigations revealed that the molar ratio of citrate ion/Bi3+ is the dominant parameter for the formation of well-structured nanoplates.
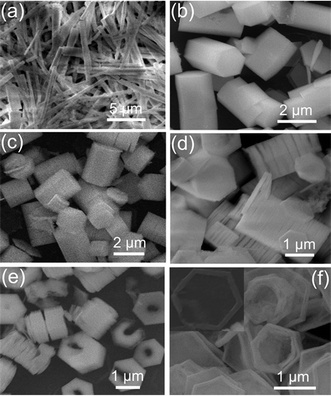 | ||
| Fig. 2 SEM images of Bi2O3 samples with typical structures in the presence of different molar ratios of citrate ion/Bi3+: (a) long nanorods, without citrate ions; (b) short microrods, molar ratio of citrate ion/Bi3+ of 0.02; (c) short and fat microrods, 0.2; (d) stacked cylinders made of several or tens of nanoplates, 2; (e) nanoplates with holes, 5; (f) hexagonal nanorings, 10. | ||
As we all know, the growth habit of crystals is related to the growth rate of various crystal faces bounding the crystal, which is affected by the intrinsic crystal structure and the external conditions including the kinetic energy barrier, temperature, time and capping molecules, and so on.28,29 Generally, the face with a higher density of surface atoms is easily blocked by the adsorption of surfactants during the crystal growth, and the growth along this facet is therefore considerably restricted. Citrate is an important biological ligand which can adsorbs strongly on metal and mineral surfaces and significantly alters the surface properties and mineral growth behavior.30,31 It has been suggested that citrate ions may bind on the (001) planes and exert strong inhibiting effects on ZnO elongation.32 We believe that a similar effect occurs during our synthesis process. The citrate molecules are selectively adsorbed on the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11) planes which have a denser surface density of atoms than those of (110), (101), and (01
11) planes which have a denser surface density of atoms than those of (110), (101), and (01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) planes, and the growth along the [
) planes, and the growth along the [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11] direction is suppressed under these conditions, but the crystals are still able to grow sideways, leading to the formation of nanoplates.
11] direction is suppressed under these conditions, but the crystals are still able to grow sideways, leading to the formation of nanoplates.
The result suggests that citrate molecules slow down the crystal growth along the [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11] orientation, which provides a simple approach to controlling the morphology and aspect ratio of the Bi2O3 nanostructures. We also investigated the effect of citrate concentrations on the crystal morphology of the obtained Bi2O3. While maintaining the amount of HMT and Bi(NO3)2·6 H2O constant, the citrate concentrations were changed to modify the product morphology, and the important results are presented in Fig. 2. Without citrate ions, long Bi2O3 rods were formed (shown in Fig. 2a). The nanorods have lengths of 6–9 μm and diameters ranging from 300 to 900 nm, producing a typical aspect ratio (height to width) of about 13. When a very small quantity of citrate ions (citrate ion/Bi3+ of 0.02) was added, the Bi2O3 rods became shorter and thicker (Fig. 2b), and the aspect ratio (height to width) was rapidly decreased. As the citrate concentration increased, the length of the Bi2O3 rods decreased further with increased diameter (Fig. 2c), and plate-like Bi2O3 nanostructures rather than rod-like crystals were obtained at a high citrate concentration (citrate ion/Bi3+ of 1), as presented in Fig. 1a. By further increasing the amount of citrate ion/Bi3+ to 2, the product retained the hexagonal plate-like shape, as seen in Fig. 2d. However, an obvious difference that can be observed is the presence of many long strings of hexagonal plates. The diameter and thickness of the plates are estimated to range from 1.3 to 1.8 μm and 70 to 130 nm, respectively. The nanoplates stacked face-to-face with each other to form long strings with a length up to a few microns, which, as a whole, resembled the cylinders. The stacked cylinders can be made of several or tens of nanoplates. A typical TEM image of the stacked cylinder is displayed in Fig. 3a. As can be seen in Fig. 3a, the interstices between neighboring nanoplates are clearly observed. The formation of these special structures of plate-built cylinders is due to the self-assembly of the primary nanoplates through oriented attachment. Similar phenomena have been demonstrated on LaF3 nanodisks and PbWO4 microcrystals.33,34 As the molar ratio of citrate ion/Bi3+ was increased to 5, besides the plate-built cylinders with thinner thickness, some nanoplates with a hole at the middle appeared, as seen in Fig. 2e and Fig. 3b–d. The sizes and shapes of the inner holes vary from plate to plate (Fig. 3b–d). Most of the plates have one hole (Fig. 3b). While in some cases, the plates may possess two or more holes (Fig. 3d). Another main feature is that the nanoplates have a wedge shape that becomes thinner towards the center (Fig. 3b–d). Further increasing the molar ratio of citrate ion/Bi3+ to 10, almost all the nanoplates were dramatically changed into ring-like structures (Fig. 2f). Most of the rings have an hexagonal base and their morphology is fairly uniform. These nanorings have outer diameters of 0.7–1.3 μm, inner diameters of 0.6–1.2 μm, and wall thickness of 30–80 nm. The hexagonal cross-section of the outer shape and central hole is easily identified (the inset in Fig. 2f). It is obvious that the nanorings are evolved from the nanoplates with holes. The formation of these nanorings can be attributed to the well-known Ostwald ripening process from the inner side toward the outside of the nanoplates. Under high concentration of citrate ions, the thickness of the formed plate is thinner and the density of defects at the center of the plate is higher due to the dense citrate ions stabilized primary nanocrystals. Therefore, the central part of the plate has a large tendency to dissolve35 and eventually leads to the formation of a hole at the center. As the hole becomes larger, the plate becomes a hexagonal ring.
11] orientation, which provides a simple approach to controlling the morphology and aspect ratio of the Bi2O3 nanostructures. We also investigated the effect of citrate concentrations on the crystal morphology of the obtained Bi2O3. While maintaining the amount of HMT and Bi(NO3)2·6 H2O constant, the citrate concentrations were changed to modify the product morphology, and the important results are presented in Fig. 2. Without citrate ions, long Bi2O3 rods were formed (shown in Fig. 2a). The nanorods have lengths of 6–9 μm and diameters ranging from 300 to 900 nm, producing a typical aspect ratio (height to width) of about 13. When a very small quantity of citrate ions (citrate ion/Bi3+ of 0.02) was added, the Bi2O3 rods became shorter and thicker (Fig. 2b), and the aspect ratio (height to width) was rapidly decreased. As the citrate concentration increased, the length of the Bi2O3 rods decreased further with increased diameter (Fig. 2c), and plate-like Bi2O3 nanostructures rather than rod-like crystals were obtained at a high citrate concentration (citrate ion/Bi3+ of 1), as presented in Fig. 1a. By further increasing the amount of citrate ion/Bi3+ to 2, the product retained the hexagonal plate-like shape, as seen in Fig. 2d. However, an obvious difference that can be observed is the presence of many long strings of hexagonal plates. The diameter and thickness of the plates are estimated to range from 1.3 to 1.8 μm and 70 to 130 nm, respectively. The nanoplates stacked face-to-face with each other to form long strings with a length up to a few microns, which, as a whole, resembled the cylinders. The stacked cylinders can be made of several or tens of nanoplates. A typical TEM image of the stacked cylinder is displayed in Fig. 3a. As can be seen in Fig. 3a, the interstices between neighboring nanoplates are clearly observed. The formation of these special structures of plate-built cylinders is due to the self-assembly of the primary nanoplates through oriented attachment. Similar phenomena have been demonstrated on LaF3 nanodisks and PbWO4 microcrystals.33,34 As the molar ratio of citrate ion/Bi3+ was increased to 5, besides the plate-built cylinders with thinner thickness, some nanoplates with a hole at the middle appeared, as seen in Fig. 2e and Fig. 3b–d. The sizes and shapes of the inner holes vary from plate to plate (Fig. 3b–d). Most of the plates have one hole (Fig. 3b). While in some cases, the plates may possess two or more holes (Fig. 3d). Another main feature is that the nanoplates have a wedge shape that becomes thinner towards the center (Fig. 3b–d). Further increasing the molar ratio of citrate ion/Bi3+ to 10, almost all the nanoplates were dramatically changed into ring-like structures (Fig. 2f). Most of the rings have an hexagonal base and their morphology is fairly uniform. These nanorings have outer diameters of 0.7–1.3 μm, inner diameters of 0.6–1.2 μm, and wall thickness of 30–80 nm. The hexagonal cross-section of the outer shape and central hole is easily identified (the inset in Fig. 2f). It is obvious that the nanorings are evolved from the nanoplates with holes. The formation of these nanorings can be attributed to the well-known Ostwald ripening process from the inner side toward the outside of the nanoplates. Under high concentration of citrate ions, the thickness of the formed plate is thinner and the density of defects at the center of the plate is higher due to the dense citrate ions stabilized primary nanocrystals. Therefore, the central part of the plate has a large tendency to dissolve35 and eventually leads to the formation of a hole at the center. As the hole becomes larger, the plate becomes a hexagonal ring.
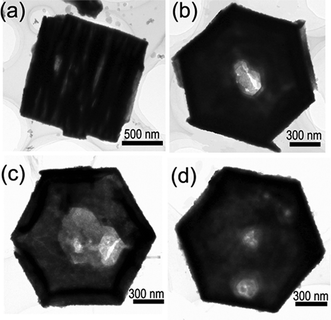 | ||
| Fig. 3 (a) Typical TEM image of the plate-built cylinder; (b–d) TEM images of the nanoplates with one or more holes. | ||
Accompanied with the morphology changes from long hexagonal rods to short hexagonal crystals, hexagonal nanoplates, and then to plate-built cylinders, nanoplates with holes, and hexagonal nanorings with the increase of citrate concentrations, the size and the aspect ratio of the crystals can also be well modulated accordingly. The aspect ratios (height to width) and widths as a function of citrate concentrations are shown in Fig. 4a and b, respectively. The results demonstrate that the aspect ratio of the crystals is directly related to the citrate concentration. The aspect ratio shifts to progressively lower values as citrate concentration increases, which can be tuned from 13 to 0.03 under current experimental conditions. The width, does not increase directly in relation to an increase in citrate concentration. As the molar ratio of citrate ion/Bi3+ increases, the width increases markedly at first and reaches a maximum value of about 2.1 μm at a molar ratio of 1, it then reduces slightly, and reaches a value of 1.2 μm at a molar ratio of 10. The results indicate that at a molar ratio of less than 1, the citrate ions mainly adsorb on the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11) facets, but with an increase in the molar ratio (>1) the growth of the ± (101), ±(110), and ± (01
11) facets, but with an increase in the molar ratio (>1) the growth of the ± (101), ±(110), and ± (01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) side facets is also suppressed by the adsorption of citrate ions, inducing the reduced width of Bi2O3 crystals. In fact, when the molar ratio is increased to 20, almost no Bi2O3 crystals are formed due to the suppressed growth of Bi2O3 crystals by the strong adsorption of citrate ions.
) side facets is also suppressed by the adsorption of citrate ions, inducing the reduced width of Bi2O3 crystals. In fact, when the molar ratio is increased to 20, almost no Bi2O3 crystals are formed due to the suppressed growth of Bi2O3 crystals by the strong adsorption of citrate ions.
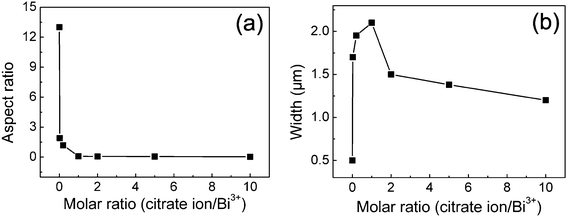 | ||
| Fig. 4 The aspect ratios (height to width) (a) and widths (b) for Bi2O3 structures as a function of citrate concentrations. | ||
The UV-vis absorption spectra were examined to study the optical properties of the representative Bi2O3 samples. As an example, Fig. 5 shows the typical absorption spectrum of the Bi2O3 nanoplates shown in Fig. 1a (denoted as sample 4). An estimate of the optical bandgap Eg for direct interband transitions can be made by using the following equation for a semiconductor:
| (αhv)2 = B(hv − Eg) |
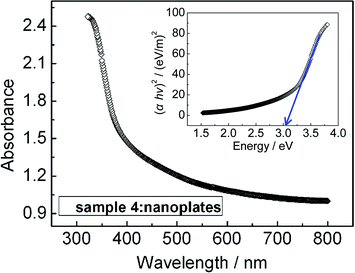 | ||
| Fig. 5 Optical absorption spectrum of the Bi2O3 nanoplates, the inset shows the plot of (αhv)2versus hv. | ||
where hv is the photon energy, α is the absorption coefficient, B is the parameter that relates to the effective masses associated with the valence and conduction bands. Therefore, the optical bandgap for the absorption edge can be obtained by extrapolating the linear portion of the plot (αhv)2versus hv to α = 0. The inset of Fig. 5 shows the plot of (αhv)2versus hv calculated from the absorption spectrum. From the results, the direct bandgap is determined to be 3.05 eV for the Bi2O3 nanoplates. Using a similar method, the direct bandgaps for the samples shown in Fig. 2 (denoted as sample 1, 2, 3, 5, 6, and 7, respectively) are determined to be 2.50, 2.57, 2.75, 3.93, 3.78, and 3.50 eV, respectively (shown in Table 1). It has been reported that the bandgap of Bi2O3 may change from 2 to 3.96 eV,19,20,36,37 depending on preparation technology. In our case, samples 1 and 2 have similar bandgaps, and the bandgaps for the samples 1–4 show a slight enhancement with the aspect ratio decreasing. As the morphologies of the samples changed from plates to plate-built cylinders and nanorings, the bandgaps show obvious variations. This indicates that the bandgaps of our samples change with the sample shapes and sizes, which may be partially associated with the modified Bi2O3 surfaces by the adsorption of citrate ions.
| Samples | Morphologies | Bandgaps/eV |
|---|---|---|
| 1 | Long nanorods | 2.50 |
| 2 | Short microrods | 2.57 |
| 3 | Short microrods | 2.75 |
| 4 | Nanoplates | 3.05 |
| 5 | Plate-built cylinders | 3.93 |
| 6 | Nanoplates with holes | 3.78 |
| 7 | Nanorings | 3.50 |
4. Conclusions
In summary, we have demonstrated a novel and simple citrate-assisted solution approach to the shape-selective synthesis of Bi2O3 nanostructures with controllable bandgaps and morphologies at low temperature. Due to the selective adsorption of the citrate molecules on certain faces during crystal growth, nanocrystals with different morphologies, such as nanorods, nanoplates, plate-built cylinders, nanoplates with holes, and nanorings, are created. The bandgaps and aspect ratios of the Bi2O3 nanostructures are easily tuned by modifying the product morphologies by adjusting the amount of trisodium citrate. The obtained hexagonal nanoplates, plate-built cylinders, and nanorings are new members in the family of Bi2O3 nanostructures. More novel Bi2O3 nanostructures with controllable morphologies and sizes can be manufactured with our method by optimizing the experimental parameters. These novel Bi2O3 nanostructures are expected to have great potential for photocatalysis, gas sensors, and photoelectrochemistry.Acknowledgements
This work was supported by the Special Prophase Program for Key Basic Research of the Ministry of Science and Technology of China (973 Program: 2010CB234606), the National Natural Science Foundation of China (Grant Nos. 11105047, 10975109 and 10575078), the Major Program of Education Commission of Hubei Province (Grant Nos. Z20101001 and Q20091007), and the Key Program of Natural Science of Hubei Province (Grant Nos. 2009CDA027 and 2009CDB351).References
- C. Burda, X. B. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS.
- L. Liao, H. B. Lu, J. C. Li, H. He, D. F. Wang, D. J. Fu, C. Liu and W. F. Zhang, J. Phys. Chem. C, 2007, 111, 1900–1903 CAS.
- E. S. Jang, J. H. Won, S. J. Hwang and J. H. Choy, Adv. Mater., 2006, 18, 3309–3312 CrossRef CAS.
- Y. F. Qiu and S. H. Yang, Adv. Funct. Mater., 2007, 17, 1345–1352 CrossRef CAS.
- S. K. Blower and C. Greaves, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1988, 44, 587–589 CrossRef.
- P. Shuk, H. D. Wiemhofer, U. Guth, W. Gopel and M. Greenblatt, Solid State Ionics, 1996, 89, 179–196 CrossRef CAS.
- K. Brezesinski, R. Ostermann, P. Hartmann, J. Perlich and T. Brezesinski, Chem. Mater., 2010, 22, 3079–3085 CrossRef CAS.
- L. Zhou, W. Z. Wang, H. L. Xu, S. M. Sun and M. Shang, Chem.–Eur. J., 2009, 15, 1776–1782 CrossRef CAS.
- X. L. Gou, R. Li, G. X. Wang, Z. X. Chen and D. Wexler, Nanotechnology, 2009, 20, 495501 CrossRef.
- F. L. Zheng, G. R. Li, Y. N. Ou, Z. L. Wang, C. Y. Su and Y. X. Tong, Chem. Commun., 2010, 46, 5021–5023 RSC.
- Z. L. Xu, I. Tabata, K. Hirogaki, K. Hisada, T. Wang, S. Wang and T. Hori, RSC Adv., 2012, 2, 103–106 RSC.
- Y. F. Qiu, M. L. Yang, H. B. Fan, Y. Z. Zuo, Y. Y. Shao, Y. J. Xu, X. X. Yang and S. H. Yang, CrystEngComm, 2011, 13, 1843–1850 RSC.
- J. In, I. Yoon, K. Seo, J. Park, J. Choo, Y. Lee and B. Kim, Chem.–Eur. J., 2011, 17, 1304–1309 CrossRef CAS.
- Y. F. Qiu, D. F. Liu, J. H. Yang and S. H. Yang, Adv. Mater., 2006, 18, 2604–2608 CrossRef CAS.
- X. P. Shen, S. K. Wu, H. Zhao and Q. Liu, Phys. E., 2007, 39, 133–136 CrossRef CAS.
- L. Liu, J. Jiang, S. M. Jin, Z. M. Xia and M. T. Tang, CrystEngComm, 2011, 13, 2529–2532 RSC.
- H. W. Kim, J. H. Myung, S. H. Shim and C. Lee, Appl. Phys. A: Mater. Sci. Process., 2006, 84, 187–189 CrossRef CAS.
- H. W. Kim, in 28th Dry Process Symposium (DPS), Elsevier Science Sa, Nagoya, JAPAN, 2006, pp. 3665–3668 Search PubMed.
- B. J. Yang, M. S. Mo, H. M. Hu, C. Li, X. G. Yang, Q. W. Li and Y. T. Qian, Eur. J. Inorg. Chem., 2004, 1785–1787 CrossRef CAS.
- L. Li, Y. W. Yang, G. H. Li and L. D. Zhang, Small, 2006, 2, 548–553 CrossRef CAS.
- H. B. Lu, S. M. Wang, B. H. Dong, Z. X. Xu, L. Zhao and J. C. Li, J. Phys. Soc. Jpn., 2010, 79, 094802 CrossRef.
- X. G. Han, M. S. Jin, S. F. Xie, Q. Kuang, Z. Y. Jiang, Y. Q. Jiang, Z. X. Xie and L. S. Zheng, Angew. Chem., Int. Ed., 2009, 48, 9180–9183 CrossRef CAS.
- H. B. Lu, S. M. Wang, L. Zhao, J. C. Li, B. H. Dong and Z. X. Xu, J. Mater. Chem., 2011, 21, 4228–4234 RSC.
- X. G. Han, Q. Kuang, M. S. Jin, Z. X. Xie and L. S. Zheng, J. Am. Chem. Soc., 2009, 131, 3152–3153 CrossRef CAS.
- M. Zhao, X. L. Chen, Y. J. Ma, J. K. Jian, L. Dai and Y. P. Xu, Appl. Phys. A: Mater. Sci. Process., 2004, 78, 291–293 CrossRef CAS.
- J. C. Yu, A. W. Xu, L. Z. Zhang, R. Q. Song and L. Wu, J. Phys. Chem. B, 2004, 108, 64–70 CrossRef CAS.
- S. S. Bhande, R. S. Mane, A. V. Ghule and S. H. Han, Scr. Mater., 2011, 65, 1081–1084 CrossRef CAS.
- S. M. Lee, S. N. Cho and J. Cheon, Adv. Mater., 2003, 15, 441–444 CrossRef CAS.
- H. B. Lu, L. Liao, H. Li, D. F. Wang, J. C. Li, Q. Fu, B. P. Zhu and Y. Wu, Phys. E., 2008, 40, 2931–2936 CrossRef CAS.
- C. Liu and P. M. Huang, Can. J. Soil Sci., 2000, 80, 445–454 CrossRef CAS.
- C. Liu and P. M. Huang, Soil Sci. Soc. Am. J., 1999, 63, 65–72 CrossRef CAS.
- J. B. Liang, J. W. Liu, Q. Xie, S. Bai, W. C. Yu and Y. T. Qian, J. Phys. Chem. B, 2005, 109, 9463–9467 CrossRef CAS.
- Y. Cheng, Y. S. Wang, Y. H. Zheng and Y. Qin, J. Phys. Chem. B, 2005, 109, 11548–11551 CrossRef CAS.
- B. Liu, S. H. Yu, L. J. Li, Q. Zhang, F. Zhang and K. Jiang, Angew. Chem., Int. Ed., 2004, 43, 4745–4750 CrossRef CAS.
- G. W. She, X. H. Zhang, W. S. Shi, X. Fan and J. C. Chang, Electrochem. Commun., 2007, 9, 2784–2788 CrossRef CAS.
- L. Leontie, M. Caraman, M. Delibas and G. I. Rusu, Mater. Res. Bull., 2001, 36, 1629–1637 CrossRef CAS.
- L. Leontie, M. Caraman, A. Visinoiu and G. I. Rusu, Thin Solid Films, 2005, 473, 230–235 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
