Oxanorbornane-based amphiphilic systems: design, synthesis and material properties†
M.
Ganesan
and
K. M.
Muraleedharan
*
Department of Chemistry, Indian Institute of Technology Madras, Chennai, 600 036, India. E-mail: mkm@iitm.ac.in; Fax: +91 44 2257 4202; Tel: +91 44 2257 4233
First published on 23rd March 2012
Abstract
The use of oxanorbornane-based sugar-like surfaces in the development of new amphiphilic systems is demonstrated. Crystallographic analysis and results from aggregation studies showed their close resemblance to glycolipids.
Glycolipids, mainly found on the outer leaflet of cell membranes, are directly involved in cell–cell interaction and recognition of extracellular factors such as antibodies, lectins and toxins.1–3 Their presence and distribution can profoundly affect membrane's stability, flexibility and reformability—properties that are crucial for events such as endocytosis and cytokinesis. The ability of extremophiles to survive under harsh environments is attributed to special glycolipid compositions of their membranes. For example, the membranes of the archaea domain are stabilized by bolaphile type of lipid components which span the membrane thickness. Such lipids also possess relatively uncommon carbohydrate moieties such as β-D-galactofuranosyl units as the head groups. Despite its lower hydrolytic stability, the reason behind the choice of the furanose form of the sugar for such environments is not completely understood.4,5 The nature and relative numbers of the head groups and hydrocarbon chains, and their orientation with respect to each other are the important factors that determine the type of mesophases possible from these amphiphiles.6–8 Can we develop amphiphilic systems which could show some of the material properties of glycolipids? While looking for suitable head groups for the design of such compounds, we became interested in the oxanorbornane-scaffold because of the possibility of introducing multiple hydroxyl groups around the core. This paper reports the synthetic details and assessment of the aggregation behaviour of this new class of amphiphilic systems.
The synthetic scheme commenced with the preparation of the meso-diol 1 (Scheme 1) from furan and maleic anhydride through a cycloaddition–reduction sequence. This was first desymmetrized using Pseudomonas Amano Lipase (PS-Amano) through trans-acetylation with vinyl acetate. The absolute stereochemistry of the resulting mono-acetylated product was verified by comparing its [α]D with the reported value.9 After silylation, the product 4 was de-acetylated and then reacted with appropriate acid chlorides to get the lipid precursors 5a–e. These compounds on OsO4 mediated cis-dihydroxylation and silyl deprotection afforded the compounds 6a–e in 90–96% yields. In the 1H NMR spectrum of 6e, as a representative example, the signals at 4.18 and 3.95 ppm were assigned to the diasterotopic protons C10–HaHb based on their HMBC correlation with ester carbonyl carbon (Fig. 1). The former, which appeared as a doublet of doublet with J values of 6.0, 11.2 Hz, had COSY correlation with C10-Hb and C2-H. The exo nature of hydroxyls and lipid chains with respect to the oxanorbornane skeleton was confirmed by NOE experiments; irradiation of the CH signal at 3.95 ppm corresponding to H5 and H6 in 6e resulted in a 3.3% increase in the intensity for H2 and H3 protons. Noticeable COSY and HMBC correlations that helped in signal assignment are shown pictorially in Fig. 1.
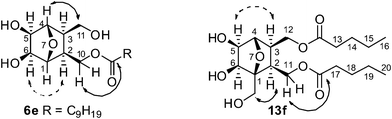 | ||
| Fig. 1 Dashed arrows connect nuclei which showed NOE on selective irradiation, and solid arrows indicate HMBC correlations; Carbon atoms are numbered arbitrarily. | ||
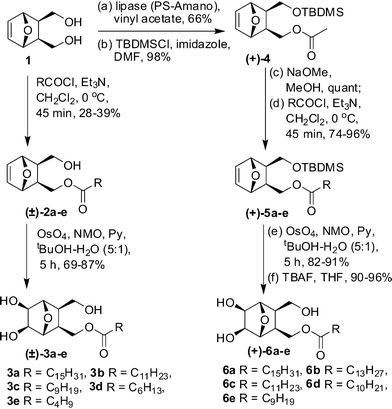 | ||
| Scheme 1 Synthesis of monoalkyl lipids (±)–3a–e and (+)–6a–e. | ||
The reaction of 1 with one equivalent of the acid chloride and cis-dihydroxylation of the resulting monohydroxy derivative led to the racemic compounds 3a–e in 69–87% yields. As in the case of 6e, NOE, observed between H2–H6 and H3–H5, supported the exo-orientation of the secondary hydroxyls and hydroxymethylene moieties with respect to the oxanorbornane skeleton.
The use of O-benzyl protected furfuryl alcohol in the cycloaddition step enabled us to prepare a related series of compounds with one head group and two lipophilic chains, as shown in Scheme 2. The racemic diol 10 obtained after the reduction of the Diels–Alder product 9 was acylated with an appropriate acid chloride and then dihydroxylated using OsO4 to get the compounds 12a–f. These, on hydrogenolysis under H2-Pd/C conditions afforded the amphiphiles 13a–f in 71–95% yields. The spectral assignment of 13f—a representative example—started taking the singlet at 4.23 ppm corresponding to the bridge-head proton C4–H as the reference. Other protons in the oxanorbornane skeleton were identified through a combination of COSY, HSQC and HMBC experiments and the most notable correlations are presented in Fig. 1. As in the case of 6e, the exo nature of hydroxyl and lipid chains with respect to the oxabicyclo ring system was confirmed by NOE experiments.
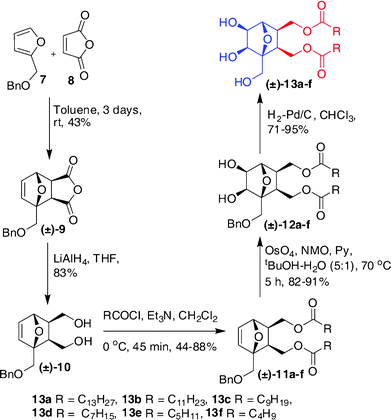 | ||
| Scheme 2 Synthesis of dialkyl lipids (±)–13a–f. | ||
Although crystallization of amphiphilic systems, in general, is a tedious task, we were successful in getting X-ray quality crystals of the compound 12e by slow evaporation of its solution in methanol–CH2Cl2 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture. They belonged to the triclinic system under the space group P
1) mixture. They belonged to the triclinic system under the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The lattice showed interdigitated molecular arrangement stabilized by hydrogen bonding between C-5 and C-6 hydroxyls from the head groups, as shown in Fig. 2. Interestingly, the aliphatic chains were found tilted ∼90° with respect to each other, retaining a bilayer type of organization in the lattice with the head groups contributing to a thickness of ∼13.4 Å. To add to our excitation, the (−) isomer of 3c also crystallized from the racemic mixture when the CDCl3 solution was allowed to slowly evaporate in a closed NMR tube at room temperature. Subsequent X-ray diffraction analysis showed them as monoclinic under the P21 the space group. In the lattice, the molecules exhibited a peculiar three-dimensional arrangement stabilized by head–head hydrogen bonding interactions and segregation of the lipid chains.
. The lattice showed interdigitated molecular arrangement stabilized by hydrogen bonding between C-5 and C-6 hydroxyls from the head groups, as shown in Fig. 2. Interestingly, the aliphatic chains were found tilted ∼90° with respect to each other, retaining a bilayer type of organization in the lattice with the head groups contributing to a thickness of ∼13.4 Å. To add to our excitation, the (−) isomer of 3c also crystallized from the racemic mixture when the CDCl3 solution was allowed to slowly evaporate in a closed NMR tube at room temperature. Subsequent X-ray diffraction analysis showed them as monoclinic under the P21 the space group. In the lattice, the molecules exhibited a peculiar three-dimensional arrangement stabilized by head–head hydrogen bonding interactions and segregation of the lipid chains.
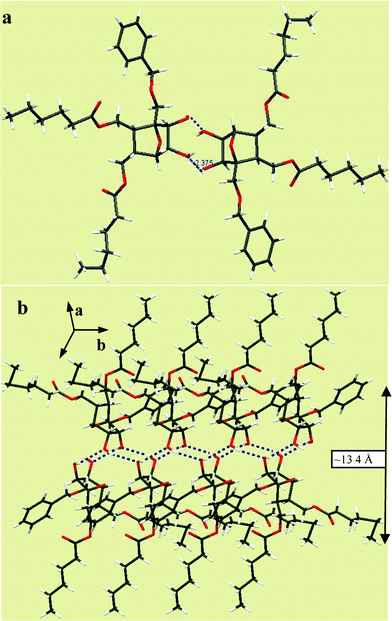 | ||
| Fig. 2 a) Crystal structure of dialkyl lipid 12e in the unit cell. b) Molecular packing of 12e in the crystal lattice showing a bilayer type of molecular arrangement. | ||
Fig. 3a shows the interdigitated arrangement wherein the primary hydroxyl group at C-3 is hydrogen bonded to the C-5 hydroxyl of the molecule in the upper layer (O⋯H–O = 2.007 Å) and the one on the lower (–OH⋯O = 1.933 Å). Such an arrangement also grew in the perpendicular direction through bifurcated hydrogen bonding involving the oxygen of primary hydroxyl and C6–OH from the neighboring layer as shown in Fig. 3b.
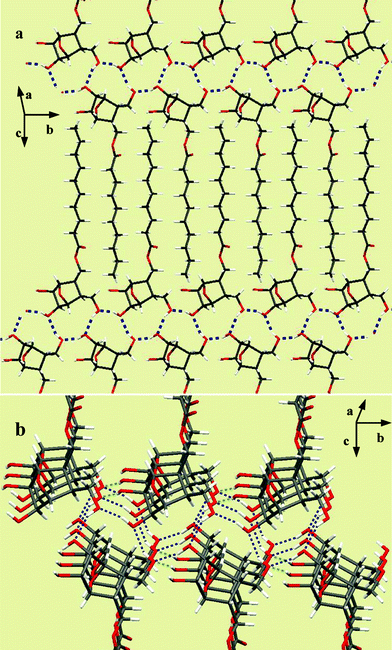 | ||
| Fig. 3 a) Assembly of 3c in the lattice involving head-head interactions. b) Growth along the perpendicular direction involving secondary interactions. | ||
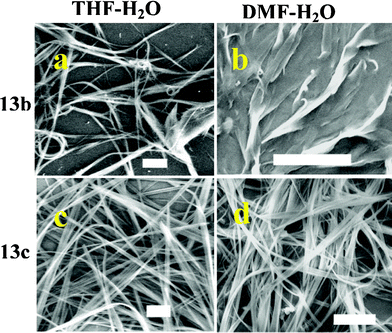
As mentioned, the nature and relative cross-sectional areas of hydrophilic and hydrophobic domains in amphiphilic systems can greatly influence their self organization. This can also be controlled externally by modulating the chemical environment such as solvent systems or other additives.10,11 In order to have a comparative assessment of the properties of amphiphiles synthesized here with that of glycolipids, their self-organization was examined by scanning electron microscopic imaging of the samples prepared under controlled conditions. When the aggregation was initiated by adding water (3 mL) to solutions of the bisalkyl lipids 13b–d in THF (1 mg mL−1), they spontaneously assembled to give wire-like superstructures. Selected images obtained from compounds 13b and 13c are shown in Fig. 4. The aggregation profile remained more or less unchanged on using polar solvents such as acetonitrile, acetone or DMF in place of THF (ESI†); the ribbon like morphologies formed from 13b in DMF–H2O were the only notable exception (Fig. 4b).
Direct drop-casting of 13d from these organic solvents led to either ribbon-like or fibrous morphologies (ESI†). The aggregation of 13c was more sensitive to the environment, which gave nanotube-like aggregates from DMF, flower-like pattern from ACN, and vesicles from THF, as shown in Fig. 5. Among these, the formation of vesicles was found to be very much dependant on the concentration of the solution and sample preparation conditions.12 We are currently working towards identifying the optimum conditions that can give such systems for detailed investigations. Since the monoalkyl lipids 6a–e did not show aggregation under the above conditions, we used a water solution of P123 ((ethylene glycol)20–(propylene glycol)70–(ethylene glycol)20) during sample preparation.
 | ||
| Fig. 5 Solvent dependant aggregation of 13c in DMF, ACN & THF solvent systems. Scale bar 10 μM. | ||
Among these, only 6a and 6b gave samples suitable for SEM imaging. In a typical procedure, 200 μL of an ACN solution of these compounds (1 mg mL−1) was added to a solution of P123 in water (5 g L−1, 1 mL) and the mixture was stirred vigorously. The mixture slowly turned into a suspension which became finer after 30 min. This was centrifuged, washed with water to remove excess P123 and the white powder was reconstituted in water. A drop of the resulting dispersion was placed on a glass plate pre-treated with piranha solution, allowed to air-dry and used for imaging. The aggregation of the monoalkyl lipids 6a and 6b was drastically different compared to that from dialkyl lipids 13b–c presented in Fig. 4 and 5. Microcrystalline assemblies with well-defined edges formed from compound 6a and presented in Fig. 6 clearly show the regulatory role of P123 during aggregation. Compound 6b with a 13-carbon chain at the same time gave rod-like structures (Fig. 6). The amphiphilic polymer P123 is known to regulate the assembly of chemical systems by preferentially blocking different faces after nucleation.10,11,13 The present observation is unique since it shows the regulation of the aggregation of one amphiphilic system by another.
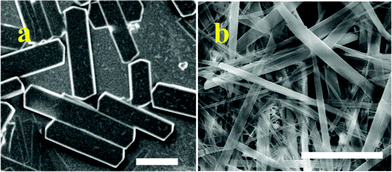 | ||
| Fig. 6 Microcrystalline assemblies of 6a & 6b. Scale bar 10 μM. | ||
Subsequently, the mesophase formation in compounds 6e and 13c was examined by optical polarization microscopic (OPM) imaging. The samples, kept in between glass plates were first heated above their melting points, and the changes during cooling was observed through a Nikon Eclipse LV 100 POL polarizing optical microscope. In general, all compounds irrespective of whether odd or even number of methylene units is present in the hydrocarbon chain, exhibited the mesogenic behaviour. Fan-like defect textures obtained from the monoalkyl lipid 6e and the dialkyl lipid 13c are shown in Fig. 7. A literature search revealed that such patterns are indicative of the Smectic A phase, widely seen in natural glycolipids and arise due to a layered arrangement.8,14,15 Detailed studies on liquid crystalline properties and thermal behaviour of this class of compounds are currently underway and the results will be reported in due course.
 | ||
| Fig. 7 OPM images of lipids a) 6e and b) 13c; magnification 20×. | ||
The aforementioned aggregation details clearly show the potential use of polyhydroxylated oxanorbornane skeletons as head-groups in lipid design. These compounds can be prepared from simple starting materials through a straightforward synthetic route. The possibility of accessing different stereoisomers of the head groups by proper choice of the starting materials or reaction conditions would enable us to get a larger group of amphiphiles for understanding their structure–property relationships. The X-ray diffraction analysis of crystals from compounds 3c and 12e showed the involvement of hydrogen bonded interactions from the head groups in stabilizing the extended assembly in 2- and 3-dimensions. In the case of 12e, the assembly was due to the interactions involving –C6–OH⋯O–C5– from molecules arranged interdigitatively. There was an angle of ∼90° between the alkyl chains which suggest that the molecular packing in this type of dialkyl lipids could be considerably different to that of monoalkyl lipids. The assembly of the monoalkyl lipid 3c was different, as anticipated, and was an extended three-dimensional array of molecules connected through hydrogen bonding between C-5-OH, C-6-OH and the primary hydroxyl group at C-3, and hydrophobic clustering in between the layers stabilized its lattice.
The dialkyl lipids 13b–c gave macromolecular wires from a biphasic medium, but ribbon- or sheet-like structures were seen when the samples were prepared by direct drop-cast of their solutions in organic solvents. This is not unusual as the extent and nature of solvation is chemical environment-dependant which would affect the molecular ordering and growth. One of the notable outcomes from this study is the formation of nanotube-like structures from the compound 13c (Fig. 5). As mentioned earlier, such systems have tremendous significance in host–guest chemistry, biological sensing, and development of mesoporous materials.
Conclusions
We have designed and synthesized a new class of amphiphilic systems with a polyhydroxylated oxanorbornane skeleton as the head group. Use of appropriate starting materials and suitable protection–deprotection procedures enabled us to prepare both monoalkyl and dialkyl lipids in acceptable yields. X-ray crystallographic analysis of representative compounds from these amphiphiles showed the involvement of primary- and secondary hydroxyl groups in propagating the molecular assembly in 2- and 3-dimensions. The resemblance of these systems to glycolipids became evident from SEM imaging studies which showed their propensity to form nanowires, nanotubes, vesicles, ribbons or discrete microcrystalline structures under different chemical environments. OPM studies confirmed their thermotropic liquid crystalline nature, and compounds 6e and 13c exhibited fan-like defect textures indicative of SmA phase which is generally seen in a number of natural glycolipids.Acknowledgements
Financial support of this work by the Department of Science and Technology, INDIA (Grants SR/S1/OC-13/2007) is gratefully acknowledged. We thank Mr. V. Ramkumar, Dept. of Chemistry (IITM) and Dr Babu Vargheese, SAIF (IITM) for their help in X-ray crystallographic analysis; Dr Edamana Prasad (IITM) for extending the facility for OPM imaging; SAIF and Dept. of metallurgy (IITM) for HRSEM. M.G. thanks IIT Madras for a research fellowship. We also thank Prof. Anju C and Prof. Loganathan D. (IITM) for giving PS-Amano lipase during our early studies.References
- N. Kojima and S. Hakomori, J. Biol. Chem., 1991, 266, 17552 CAS.
- M. M. Burger, R. S. Turner, W. J. Kuhns and G. Weinbaum, Philos. Trans. R. Soc. London, Ser. B, 1975, 271, 379 CrossRef CAS.
- H. Lis and N. Sharon, Chem. Rev., 1998, 98, 637 CrossRef CAS.
- R. Auzely-Velty, T. Benvegnu, D. Plusquellec, G. Mackenzie, J. A. Haley and J. W. Goodby, Angew. Chem., Int. Ed., 1998, 37, 2511 CrossRef CAS.
- T. Benvegnu, L. Lemiegre and S. Cammas-Marion, Eur. J. Org. Chem., 2008, 4725 CrossRef CAS.
- V. Vill and R. Hashim, Curr. Opin. Colloid Interface Sci., 2002, 7, 395 CrossRef CAS.
- T. Shimizu, M. Masuda and H. Minamikawa, Chem. Rev., 2005, 105, 1401 CrossRef CAS.
- J. W. Goodby, Liq. Cryst., 2006, 33, 1229 CrossRef CAS.
- C. Cinquin, I. Schaper, G. Mandville and R. Bloch, Synlett, 1995, 339 CrossRef CAS.
- S. J. Lee, J. T. Hupp and S. T. Nguyen, J. Am. Chem. Soc., 2008, 130, 9632 CrossRef CAS.
- X. Zhang, C. Dong, J. A. Zapien, S. Ismathullakhan, Z. Kang, J. Jie, X. Zhang, J. C. Chang, C.-S. Lee and S.-T. Lee, Angew. Chem., Int. Ed., 2009, 48, 9121 CrossRef CAS.
- C. Park, I. H. Lee, S. Lee, Y. Song, M. Rhue and C. Kim, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 1199 CrossRef CAS.
- S. Kwon, A. Jeon, S. H. Yoo, I. S. Chung and H.-S. Lee, Angew. Chem., Int. Ed., 2010, 49, 8232 CrossRef CAS.
- J. W. Goodby, V. Goertz, S. J. Cowling, G. Mackenzie, P. Martin, D. Plusquellec, T. Benvegnu, P. Boullanger, D. Lafont, Y. Queneau, S. Chambert and J. Fitremann, Chem. Soc. Rev., 2007, 36, 1971 RSC.
- F. Dumoulin, D. Lafont, P. Boullanger, G. Mackenzie, G. H. Mehl and J. W. Goodby, J. Am. Chem. Soc., 2002, 124, 13737 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC reference numbers 812794 and 846065. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra01198k |
| This journal is © The Royal Society of Chemistry 2012 |