Catalyst-and solvent-free one-pot synthesis of some novel polyheterocycles from aryldiazenyl salicylaldehyde derivatives†
Narsidas J.
Parmar
*a,
Rikin A.
Patel
a,
Shashikant B.
Teraiya
a,
Deepak
Sharma
b and
Vivek K.
Gupta
b
aDepartment of Chemistry, Sardar Patel University, Vallabh Vidyanagar-388120. Dist. Anand, Gujarat, India. E-mail: njpchemdeptspu@yahoo.co.in
bPost-Graduate Department of Physics, University of Jammu, Jammu Tawi-180 006, India
First published on 21st February 2012
Abstract
A catalyst-and solvent-free domino/Knoevenagel-hetero-Diels–Alder (DKHDA) reaction of two aldyhyde substrates, allyl/prenyl ether tethered aryldiazenylsalicylaldehydes with corresponding pyrazolones and heterocyclic 1,3-diketones is described. Subsequent reduction of aryldiazenylpolyheterocycles, thus obtained in high yields (70–86%), afforded analogues amino frameworks with anticipated biological activity. While in a conventional procedure, no reaction was observed without activation of an allyl-based substrate, an excellent yield was achieved at higher temperature. For a prenyl based substrate, however it underwent smoothly to form a desired cyclised product. The stereochemistry of the compound was confirmed by various NMR experiments and a single crystal X-ray diffraction analysis.
Introduction
In today's challenging complex heterocycle preparations,1 a domino strategy is a highly efficient route to assess a wide range of polycyclic compounds.2–3 On one hand the bond forming economy and stereo selectivity are more environment-friendly approaches, and the structure suitability with general applications is a main advantage of such cascade reactions on the other hand.2aA domino/Knoevenagel–hetero-Diels–Alder (DKHDA) approach has particularly evolved as an efficient route to many bioactive natural and unnatural compounds.2a–c,4 Ring systems constructed so far include chromenopyran, pyrimidonedione, tetrahydroquinoline, benzopyrano-fused benzoprane, benzopyrano-fused napthopyran and pyranoxanthene.2a–d,4f,5
The pyrano[2, 3-c]pyrazole unit has gained much prominence since it forms a central skeleton of many compounds which are known for their antimicrobial,6 insecticidal,7 anti-inflammatory,8 and molluscicidal activity.9 While a benzopyran ring system forms a core structure of many photochromic compounds, finding practical applications in data storage, optical filters, displays, sensor protection, waveguides, and ophthalmic plastic lenses,10 one of its class called aminochromene is the precursor to a wide range of bioactive compounds.11
Also, annulations of aminochromene with heterocycles offer an interesting and useful way afford medicinal compounds.12
In view of these, it is of interest to incorporate all these basic units into polyheterocycles. So, we decided to afford chromeno-annulated pyrano-fused heterocycles, bearing an amino group or their precursors. Furthermore, many reports exist on prenyl ether-tethered aryl or heteroaryl aldehydes, but very little on the less reactive allyl dienophile.13 As a part of our previous work, we exploited some less reactive allyl-based substrates in refluxing solvent.13d Nevertheless, the more evident improvements like reduced time of reaction, cleaner reaction due to fewer side reactions and of course no use of solvent make our present protocol more economic and environmentally friendly.
In the present work, we report the synthesis of some novel benzopyrane-annulated heterocycles from an allyl-based substrate via DKHDA reaction under catalyst-and solvent-free environment. Besides the synthesis of pyrazolones, we also extended this protocol to assemble other heterocyclic-1,3-diketones. An excellent yield was achieved in a relatively shorter time with the ease of a simple work-up procedure. All the synthesized compounds could be employed as templates for screening for biological activity.
Results and discussion
Solvent-free thermal processes are important transformations14 in terms of a reaction's yields, rates and safety aspects.15 Limited molecular movements and different interactions in such a micro-environment can sometimes change the reaction mechanism and selectivity dramatically.15a Therefore, we desired to find in the present work the solvent-free thermal reaction of less active allyl based aldehyde substrates 3a–b as well as some active prenyl based substrates 3c–d. The reaction was also examined and compared with a conventional procedure.O-Allylated (3a–b)/prenylated (3c–d)-5-aryl diazenylsalicyl aldehydes were obtained from 2a–b, following a reported method (Scheme 1).16–17 DKHDA reactions of substrates 3a and 3c with 4a and 4d respectively were investigated as models to optimize the conditions. Several parameters like temperature, different refluxing solvents, microwave irradiation and with or without use of a common catalyst mediated thermal procedures were employed to evaluate the present methodology (Table 1).
 | ||
| Scheme 1 Reagents & conditions: (i) NaNO2, HCl, 0–5 °C, (ii) 10% NaOH, salicylaldehyde, 0–5 °C, (iii) K2CO3, DMF, allyl/prenyl bromide, 12 h. | ||
| Entry | Substrate | Solvent | Catalyst (mol%) | Timea | Temp. | Yield (%) |
|---|---|---|---|---|---|---|
| a Observed in the case of both the substrates, 3a and 3c. b For 3a (2 h and 140 °C for 3c). c From 3a. d From 3c, RT: room temperature, EDDA: ethylenediaminediacetate [or as a mixture with TEA: triethylamine], TBA-HS: tetrabutylammonium hydrogen sulfate. | ||||||
| 1 | 3a/3c | Acetonitrile | — | 24 h | RT | —/— |
| 2 | 3a/3c | Acetonitrile | — | 10 h | Reflux | —/Traced |
| 3 | 3a/3c | Acetonitrile | EDDA(20) | 12 h | RT | —/— |
| 4 | 3a/3c | Toluene | — | 6 h | Reflux | —/— |
| 5 | 3a/3c | Xylene | EDDA(20) | 8 h | Reflux | Tracec/82d |
| 6 | 3a/3c | Acetonitrile | ZnCl2 (25) | 8 h | Reflux | —/65d |
| 7 | 3a/3c | Acetonitrile | TBA-HS (20) | 9 h | Reflux | Tracec/70d |
| 8 | 3a | Water | Piperidine | 6 h | RT | —/— |
| 9 | 3a | Microwave | — | 7 min | — | —/— |
| 10 | 3a/3c | Solvent free | — | 4 hb | 180 °Cb | 78c/84d |
The results of various conditions applied to DKHDA approach are summarized in Table 1. In a conventional procedure, while in the absence (entries 1, 2, and 4) and presence (entries 3, and 5–8) of a catalyst/catalytic mixture, substrate 3a gave only a Knoevenagel intermediate (entries 1–9) and except for a few conditions (entries 1, 8, and 9), an excellent yield was achieved under solvent-and catalyst-free thermal reaction (entry 10). The reaction completed in almost less than half the reaction time compared to the reported method,2a,13d making it more advantageous over the conventional method. Other catalytic conditions gave no or only trace products (entries 3, 5, and 6–8). Further increase in the temperature, however, could not improve the result. Interestingly, it improved both the reaction time and temperature in the case of a prenyl-based substrate. So, this was considered an optimal condition for other domino products 7a–p (Scheme 2, Table 2). Results showed all pyrazolones afforded DKHDA cyclised products except pyrazolylarylsulphonic acids that could not yield quantitative products 7q–r (entries 17 and 18, Table 2). Instead, a black residue left on prolonged heating contained only non-separable traces of a domino product as monitored by TLC. The relatively higher temperatures required for 3a are ascribed to a larger energy gap between the HOMO (higher occupied molecular orbital's) of the allylic dienophile and the LUMO (lowest unoccupied molecular orbitals) of the Knoevenagel heterodiene in 3a. The results are well supported by a frontier orbital theory.
 | ||
| Scheme 2 Synthesis of aryldiazenylchromeno-annulated pyrano-fused pyrazoles 7a–p (i) solvent-free, 180 °C. | ||
| Entry | Product | Ar | R1 | R2 | R | Yield (%) | Time (h) | M.P. (°C)a |
|---|---|---|---|---|---|---|---|---|
| a Uncorrected. | ||||||||
| 1 | 7a | C6H5 | CH3 | C6H5 | H | 78 | 4.0 | 190–192 |
| 2 | 7b | C6H5 | CH3 | 3-Cl-C6H4 | H | 75 | 4.2 | 191–193 |
| 3 | 7c | C6H5 | C6H5 | C6H5 | H | 80 | 4.1 | 202–204 |
| 4 | 7d | C6H5 | CH3 | 2,5-Cl2-C6H3 | H | 83 | 5.0 | 96–98 |
| 5 | 7e | C6H5 | CH3 | 4-CH3-C6H4 | H | 85 | 4.0 | 179–181 |
| 6 | 7f | C6H5 | CH3 | 2-Cl-C6H4 | H | 82 | 5.1 | 114–115 |
| 7 | 7g | 4-Cl-C6H4 | CH3 | C6H5 | H | 80 | 4.5 | 219–221 |
| 8 | 7h | 4-Cl-C6H4 | CH3 | 3-Cl-C6H4 | H | 84 | 4.7 | 180–181 |
| 9 | 7i | 4-Cl-C6H4 | C6H5 | C6H5 | H | 86 | 5.0 | 209–210 |
| 10 | 7j | 4-Cl-C6H4 | CH3 | 2,5-Cl2-C6H3 | H | 70 | 5.7 | 222–224 |
| 11 | 7k | 4-Cl-C6H4 | CH3 | 4-CH3-C6H4 | H | 70 | 4.2 | 175–176 |
| 12 | 7l | 4-Cl-C6H4 | CH3 | 2-Cl-C6H4 | H | 73 | 4.4 | 108–110 |
| 13 | 7m | C6H5 | CH3 | 2-Cl-C6H4 | CH3 | 80 | 2.5 | 212–214 |
| 14 | 7n | C6H5 | CH3 | 2,5-Cl2-C6H3 | CH3 | 84 | 2.0 | 244–246 |
| 15 | 7o | 4-Cl-C6H4 | CH3 | 2-Cl-C6H4 | CH3 | 79 | 2.1 | 118–120 |
| 16 | 7p | 4-Cl-C6H4 | CH3 | 2,5-Cl2-C6H3 | CH3 | 75 | 2.4 | 276–278 |
| 17 | 7q | C6H5 | CH3 | 3-SO3H-C6H4 | H | — | 6.0 | — |
| 18 | 7r | C6H5 | CH3 | 4-SO3H-C6H4 | H | — | 6.0 | — |
1H NMR of 7a shows a doublet in the δ 4.6–4.8 Hz range with a J value lying in the 2.0–4.8 Hz range, which is attributable to benzylic methane protons on a pyranyl ring suggesting its cis-geometry. The IR band centred around 1385 cm−1 infers the presence of an azo group. The absorption bands appearing in the 270–352 nm range indicate a trans-aryldiazenyl moiety. The stereochemical outcome of the domino product was confirmed by 2D NMR experiments; nuclear Overhauser effect spectroscopy (nOes) and the double quantum filtered correlation spectroscopy (DQFCOSY). The proposed cis-geometry of bridging protons in compound 7a is fully agreed with 2D NMR experimental results (Fig. 1). The same was unambiguously confirmed by a single-crystal XRD analysis of representative compound 7a (Fig. 2–3). The crystallographic experimental data are summarized in Table 4. Packing arrangement of the molecules viewed down the a-axis is shown in Fig. 3. The crystallizing solvent chloroform is also shown along with a crystal structure of compound 7a.
 | ||
| Fig. 1 Characteristic nOes of 7a. | ||
 | ||
| Fig. 2 ORTEP diagram of compound 7a. | ||
 | ||
| Fig. 3 The packing arrangement of molecules 7a. | ||
The orientation and attack of dienophiles determine the stereochemistry of the reaction products. Accordingly, we can assume four possible transition structures.3b The exo-E-anti and endo-Z-anti would lead to a trans-adduct while the endo-E-syn and exo-Z-syn to a cis-one. In the present work, the endo-E-syn transition structure seems to be affording exclusively a cis-product even though two paths are possible (Scheme 3).
 | ||
| Scheme 3 Mechanism of DKHDA reaction; solvent-free 180 °C. | ||
The scope of the present method was further extended to other heterocyclic diketones such as N-phenyl/methyl-4-hydroxy-2-quinolone, 4-hydroxy coumarin and Meldrum’s acid (Scheme 3). All heterocyclic diketones except meldrum acid gave the desired cyclised products 9b–d, and 9f–h in high yields (Table 3). In case of meldrum acid, Knoevenagel condensate formed a ketene via retro-Diels–Alder reaction which after water trapping, converted into a β-ketoacid. Finally, its decarboxylation yielded products 9a and 9e (Scheme 3).
| Entry | Product | Ar | X | Yield (%) | Time (h) | M.P. (°C)a |
|---|---|---|---|---|---|---|
| a Uncorrected. | ||||||
| 1 | 9a | C6H5 | — | 78 | 4.2 | 195–196 |
| 2 | 9b | C6H5 | O | 70 | 4.1 | 210–212 |
| 3 | 9c | 4-Cl-C6H4 | N–CH3 | 72 | 4.5 | 240–241 |
| 4 | 9d | 4-Cl-C6H4 | N–Ph | 76 | 4.7 | 220–223 |
| 5 | 9e | 4-Cl-C6H4 | — | 73 | 4.3 | 212–214 |
| 6 | 9f | 4-Cl-C6H4 | O | 78 | 4.4 | 210–211 |
| 7 | 9g | C6H5 | N–CH3 | 77 | 4.1 | 220–222 |
| 8 | 9h | C6H5 | N–CH3 | 72 | 4.3 | 230–231 |
For our new interesting approach, we exploited and extended present methodology to assess amino benzopyran-annulated pyrano-fused heterocycles. Accordingly, we were able to synthesize some novel aminochromene-annulated ring systems 10 from 7avia reduction of the diazenyl group (Scheme 4). The formation of 10 was also confirmed by spectroscopic data. Further useful derivatization is in progress.
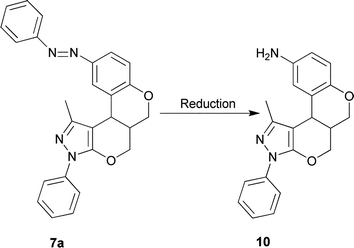 | ||
| Scheme 4 The reduction of diazenyl group and formation of amino benzopyran 10; reagent and condition: conc. HCl, SnCl2, 100 ˚C. | ||
Experimental
General
All the compounds were characterized based on elemental analysis by Perkin-Elmer 2400 Series-II elemental analyzer, ESI mass spectra by SHIMADZU LCMS-2010 spectrometer and melting points, which are uncorrected, by open capillary tube TEMPO melting point apparatus. All the reactions were monitored by silica gel 60 F254 coated thin layer chromatography (TLC) plates (Merck). The structure and stereochemistry all the compounds were confirmed by 1H NMR and 13C NMR spectra, 1H–1H COSY, NOESY spectra on BRUKER Avance (1H: 400 MHz, 13C: 100 MHz), using CDCl3 as both solvent and reference. IR spectra were recorded on a SHIMADZU FT-IR 8300 spectrophotometer, using KBr disc. UV-Visible spectra were taken on SHIMADZU UV-160 A spectrophotometer. Single crystal X-ray data were collected on Bruker CCD area-detector diffractometer equipped with graphite monochromated Mo-Kα radiation (λ = 0.71073 Å) (Table 4). The structure was solved by direct methods using SHELXS97.18 All non-hydrogen atoms of the molecule were located in the best E-map.| CCDC No | 820934 |
| Crystal description | brown plate |
| Crystal size | 0.3 × 0.2 × 0.1 mm |
| Empirical formula | C26H22N4O2·CHCl3 |
| Formula weight | 541.84 |
| Radiation, Wavelength | Mo-Kα, 0.71073 Å |
| Unit cell dimensions | a = 8.0866(3), b = 12.1875(6), c = 13.9665(7) Å |
| α = 83.079(4)° β = 80.504(4)° γ = 74.566(4)° | |
| Crystal system | Triclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| Unit cell volume | 1304.40(10) Å3 |
| Density (calculated) | 1.380 mg m−3 |
| No. of molecules per unit cell, Z | 2 |
| Temperature | 293(2) K |
| Absorption coefficient (μ) | 0.384 mm−1 |
| Absorption correction | ψ-scan (Tmin = 0.91948 and Tmax = 1.00000) |
| Extinction coefficient | 0.004(2) |
| F(000) | 560 |
| Refinement of unit cell | 4556 reflections (3.50 < θ < 28.84°) |
| Scan mode | phi and omega scan |
| θ range for entire data collection | 3.56 < θ < 25.00° |
| Reflections collected/unique | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 791/4535 791/4535 |
| Reflections observed (I > 2σ(I)) | 3089 |
| Range of indices | h = −9 to 9, k = −14 to 14, l = −16 to16 |
| R int | 0.0237 |
| R σ | 0.0332 |
| Structure determination | Direct methods |
| Refinement | Full-matrix least-squares on F2 |
| No. of parameters refined | 358 |
| Final R | 0.0603 |
| wR(F2) | 0.1572 |
| Weight | 1/[σ2(Fo2) + (0.1085P)2 + 0.2339P] |
| where P = [Fo2 + 2Fc2]/3 | |
| Goodness-of-fit | 1.006 |
| (Δ/σ)max in the final cycle | 0.001 (for U33 C11a) |
| Final residual electron density | −0.302 < Δρ < 0.510 eÅ−3 |
| Measurement | Bruker SMART CCD area detector |
| Diffractometer | |
| Software for structure solution | SHELXS97 (Sheldrick, 1997) |
| Software for refinement | SAINT 6.45(Bruker, 2003) |
| Software for molecular plotting | ORTEP-3 for (WingX), PLATON (Spek, 1999) |
| Software for geometrical calculations | PARST (Nardelli, 1995), PLATON (Spek, 2003) |
General procedure for domino reaction
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) eluent, affording compounds 7a–p or 9a–h in pure product with excellent yields. All the products were characterized based on their elemental, mass, UV-visible NMR and IR spectroscopy.
7) eluent, affording compounds 7a–p or 9a–h in pure product with excellent yields. All the products were characterized based on their elemental, mass, UV-visible NMR and IR spectroscopy.
Selected spectroscopic data of compounds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 721), 270 (12
721), 270 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 552); νmax/cm−1 = 2957, 2927, 1490, 1467, 1241, 1092, 1026, 831, 758; 1H NMR (CDCl3, 400 MHz): δ = 2.42 (s, 3H, Me), 2.60 (s, 1H, C(5a)H), 4.16 (t, 1H, Jb = 10.4 Hz, C(6)H), 4.33 (d, 1H, J = 4.8 Hz, C(11b)H), 4.43 (m, 2H, C(5)H), 4.65 (dd, 1H, J = 2.4 Hz, C(6)H), 6.99 (d, 1H, J = 10.0 Hz, ArH), 7.22–7.87 (m, 12H ArH); 13C NMR (CDCl3, 100 MHz): δ = 14.41 (CH3), 29.51 (CH, benzylic methane), 30.12 (CH, benzylic methane), 66.20 (CH2), 68.52 (CH2), 118.10, 120.34, 122.73, 123.65, 125.66, 126.04, 129.56, 129.86, 131.36 (ArCH), 99.48, 125.15, 138.65, 145.11, 146.49, 146.77, 148.09, 149.24, 149.95, 152.40, 152.81 (ArC); m/z (ESI) 423.1 [M + H+]. Anal. Calcd. for C27H23Cl3N4O2 (541.86): C, 59.85; H, 4.28; N, 10.34. Found: C, 61.01.; H, 4.80; N, 10.56;
552); νmax/cm−1 = 2957, 2927, 1490, 1467, 1241, 1092, 1026, 831, 758; 1H NMR (CDCl3, 400 MHz): δ = 2.42 (s, 3H, Me), 2.60 (s, 1H, C(5a)H), 4.16 (t, 1H, Jb = 10.4 Hz, C(6)H), 4.33 (d, 1H, J = 4.8 Hz, C(11b)H), 4.43 (m, 2H, C(5)H), 4.65 (dd, 1H, J = 2.4 Hz, C(6)H), 6.99 (d, 1H, J = 10.0 Hz, ArH), 7.22–7.87 (m, 12H ArH); 13C NMR (CDCl3, 100 MHz): δ = 14.41 (CH3), 29.51 (CH, benzylic methane), 30.12 (CH, benzylic methane), 66.20 (CH2), 68.52 (CH2), 118.10, 120.34, 122.73, 123.65, 125.66, 126.04, 129.56, 129.86, 131.36 (ArCH), 99.48, 125.15, 138.65, 145.11, 146.49, 146.77, 148.09, 149.24, 149.95, 152.40, 152.81 (ArC); m/z (ESI) 423.1 [M + H+]. Anal. Calcd. for C27H23Cl3N4O2 (541.86): C, 59.85; H, 4.28; N, 10.34. Found: C, 61.01.; H, 4.80; N, 10.56;
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 628), 271 (15
628), 271 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 803); νmax/cm−1 = 2950, 2930, 2890, 1490, 1481, 1235, 1092, 1089, 1030, 898, 822, 763; 1H NMR (CDCl3, 400 MHz): δ = 2.57 (m, 4H, Me, C(5a)H), 4.24 (d, 1H, J = 4.8 Hz, C(11b)H), 4.35 (t, 1H, Jb = 10.8 Hz, C(6)H), 4.47 (m, 2H, C(5)H), 4.57 (dd, 1H, J = 2.8 Hz, C(6)H), 6.95 (d, 1H, J = 8.4 Hz, ArH), 7.18–7.94 (m, 11H, ArH); 13C NMR (CDCl3, 100 MHz): δ = 14.12 (CH3), 30.11 (CH, benzylic methane), 30.77 (CH, benzylic methane), 66.12 (CH2), 68.49 (CH2), 117.72, 118.19, 120.29, 122.67, 123.37, 125.69, 125.84, 129.03, 130.00, 130.53 (ArCH), 99.42, 123.60, 134.69, 139.23, 147.21, 147.61, 149.41, 152.62, 154.88 (ArC); m/z (ESI) 457.1 [M + H+]; Anal. Calcd. for C26H21N4O2Cl (456.92): calcd. C 68.34, H 4.63, N 12.26; found C 68.25, H 4.58, N 12.06.
803); νmax/cm−1 = 2950, 2930, 2890, 1490, 1481, 1235, 1092, 1089, 1030, 898, 822, 763; 1H NMR (CDCl3, 400 MHz): δ = 2.57 (m, 4H, Me, C(5a)H), 4.24 (d, 1H, J = 4.8 Hz, C(11b)H), 4.35 (t, 1H, Jb = 10.8 Hz, C(6)H), 4.47 (m, 2H, C(5)H), 4.57 (dd, 1H, J = 2.8 Hz, C(6)H), 6.95 (d, 1H, J = 8.4 Hz, ArH), 7.18–7.94 (m, 11H, ArH); 13C NMR (CDCl3, 100 MHz): δ = 14.12 (CH3), 30.11 (CH, benzylic methane), 30.77 (CH, benzylic methane), 66.12 (CH2), 68.49 (CH2), 117.72, 118.19, 120.29, 122.67, 123.37, 125.69, 125.84, 129.03, 130.00, 130.53 (ArCH), 99.42, 123.60, 134.69, 139.23, 147.21, 147.61, 149.41, 152.62, 154.88 (ArC); m/z (ESI) 457.1 [M + H+]; Anal. Calcd. for C26H21N4O2Cl (456.92): calcd. C 68.34, H 4.63, N 12.26; found C 68.25, H 4.58, N 12.06.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 738), 275 (23
738), 275 (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353); νmax/cm−1 2957, 2920, 2890, 1491, 1471, 1238, 1094, 1028, 888, 817, 760; 1H NMR (CDCl3, 400 MHz): δ = 2.12 (m, 1H, C(5a)H), 4.42–4.62 (m, 5H), 6.92–8.06 (m, 18H, ArH); 13C NMR (CDCl3, 100 MHz): δ = 30.75 (CH, benzylic methane), 31.09 (CH, benzylic methane), 66.64 (CH2), 68.00 (CH2), 117.38, 121.14, 122.58, 123.75, 125.64, 126.21, 127.33, 128.34, 128.84, 128.93, 128.96, 130.31 (ArCH), 98.44, 123.78, 134.13, 138.31, 147.42, 148.85, 149.81, 152.54, 154.55 (ArC); m/z (ESI) 485.0 [M + H+]; Anal. Calcd. for C31H24N4O2 (484.55): calcd. C 76.84, H 4.99, N 11.56; found C 76.70, H 4.87, N 11.48.
353); νmax/cm−1 2957, 2920, 2890, 1491, 1471, 1238, 1094, 1028, 888, 817, 760; 1H NMR (CDCl3, 400 MHz): δ = 2.12 (m, 1H, C(5a)H), 4.42–4.62 (m, 5H), 6.92–8.06 (m, 18H, ArH); 13C NMR (CDCl3, 100 MHz): δ = 30.75 (CH, benzylic methane), 31.09 (CH, benzylic methane), 66.64 (CH2), 68.00 (CH2), 117.38, 121.14, 122.58, 123.75, 125.64, 126.21, 127.33, 128.34, 128.84, 128.93, 128.96, 130.31 (ArCH), 98.44, 123.78, 134.13, 138.31, 147.42, 148.85, 149.81, 152.54, 154.55 (ArC); m/z (ESI) 485.0 [M + H+]; Anal. Calcd. for C31H24N4O2 (484.55): calcd. C 76.84, H 4.99, N 11.56; found C 76.70, H 4.87, N 11.48.
Conclusion
In summary, we have demonstrated an efficient solvent-free thermal procedure for the one-pot synthesis of polyheterocycle via an intramolecular domino reaction. Out of the two types of aldehyde substrates employed, one contains unactivated type allyl dienophile and the second one an activated prenyl type dienophile. The former one underwent DKHDA reaction at a higher temperature. The findings highlighted the solvent-, and catalyst-free thermal procedure as more efficient than the conventional method.Acknowledgements
We sincerely express our thanks to Head, Department of Chemistry, Sardar Patel University for providing necessary research facility. Two of us (R.A.P. and S.B.T.) are grateful to UGC, New Delhi for financial assistance under the UGC scheme of RFSMS. We also thank Vaibhav Analytical Lab., Ahmadabad and Nutan Dye Chem., Surat for their time and valuable services.References
- (a) K. C. Nocolaou, S. A. Synder, T. Montagnon and G. V. Nakis, Angew. Chem., Int. Ed., 2002, 41, 1668–1698 CrossRef; (b) K. Takao, R. Munakata and K. Tadano, Chem. Rev., 2005, 105, 4779–4807 CrossRef CAS; (c) S. K. Bur and A. Padwa, Chem. Rev., 2004, 104, 2401–2432 CrossRef CAS; (d) F. Chan and J. Huang, Chem. Rev., 2005, 105, 4671–4706 CrossRef; (e) I. Coldham and R. Hufton, Chem. Rev., 2005, 105, 2765–2810 CrossRef CAS; (f) T. Hudlicky, Chem. Rev., 1996, 96, 3–30 CrossRef CAS; (g) K. C. Majumdar, A. Taher and S. Ponra, Tetrahedron Lett., 2010, 51, 147–150 CrossRef CAS; (h) V. S. Matiychuk, R. B. Lesyk, M. D. Obushak, A. Gzella, D. V. Atamanyuk, Y. V. Ostapiuk and A. P. Kryshchyshyn, Tetrahedron Lett., 2008, 49, 4648–4651 CrossRef CAS; (i) A. O. Bryhas, Y. I. Horak, Y. V. Ostapiuk, M. D. Obushak and V. S. Matiychuk, Tetrahedron Lett., 2011, 52, 2324–2326 CrossRef CAS.
- (a) L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS; (b) L. F. Tietze and U. Beifuss, Angew. Chem., Int. Ed. Engl., 1993, 32, 131–163 CrossRef; (c) H. Pellissier, Tetrahedron, 2006, 62, 1619–1665 CrossRef CAS; (d) H. Pellissier, Tetrahedron, 2006, 62, 2143–2173 CrossRef CAS; (e) L. F. Tietze and N. Rackelmann, Pure Appl. Chem., 2004, 76, 1967–1983 CrossRef CAS; (f) L. F. Tietze, T. Kinzel and C. C. Brazel, Acc. Chem. Res., 2009, 42, 367–378 CrossRef CAS; (g) D. Enders, C. Wang and J. W. Bats, Angew. Chem., Int. Ed., 2008, 47, 7539–7542 CrossRef CAS.
- (a) Z. Zhang, Q. Zhang, S. Sun, T. Xiong and Q. Liu, Angew. Chem., Int. Ed., 2007, 46, 1726–1729 CrossRef CAS; (b) D. Enders, C. Grondal and M. R. M. Hutt, Angew. Chem., Int. Ed., 2007, 46, 1570–1581 CrossRef CAS; (c) A. K. Yadav, S. Peruncheralathan, H. Ila and H. Junjappa, J. Org. Chem., 2007, 72, 1388–1394 CrossRef CAS.
- (a) E. Ceulemens, M. Voets, S. Emmers, K. Uytterhoeven, L. V. Meerrelt and W. Dehan, Tetrahedron, 2002, 58, 531–544 CrossRef; (b) N. T. Dat, J. Lee, K. Lee, Y. Hong, Y. H. Kim and J. J. Lee, J. Nat. Prod., 2008, 71, 1696–1700 CrossRef; (c) D. J. Kwok, R. N. Farr and D. J. Daves, J. Org. Chem., 1991, 56, 3711–3713 CrossRef CAS; (d) B. B. Sinder and Q. J. Lu, J. Org. Chem., 1996, 61, 2839–2844 CrossRef; (e) H. N. Borah, M. L. Deb, R. C. Boruach and P. Bhuyan, Tetrahedron Lett., 2005, 46, 3391–3393 CrossRef CAS; (f) L. F. Tietze, C. Ott, H. H. Geibler and F. Haunert, Eur. J. Org. Chem., 2001, 1625–1630 CrossRef CAS; (g) L. F. Tietze, S. Brand, T. Brumy and J. Fenner, Angew. Chem., Int. Ed. Engl., 1990, 29, 665–667 CrossRef; (h) B. B. Sinder and Q. Lu, J. Org. Chem., 1994, 59, 8065–8070 CrossRef; (i) K.C. Majumdar, A. Taher and K. Ray, Tetrahedron Lett., 2009, 50, 3889–3891 CrossRef CAS.
- (a) L. F. Tietze, U. Beifuss, M. Lokos, R. Matthias, A. Gohrt and G. M. Scheldrick, Angew. Chem., Int. Ed. Engl., 1990, 29, 527–529 CrossRef; (b) Y. R. Lee, Y. M. Kim and S. H. Kim, Tetrahedron, 2009, 65, 101–108 CrossRef CAS; (c) G. Sabitha, E. V. Reddy, N. Fatima, J. S. Yadav, K. V. S. Krisna and A. C. Kunwar, Synthesis, 2004, 1150–1154 CrossRef CAS; (d) L. F. Tietze, J. Bachmann, J. Wichmann and O. Buzkhardt, Synthesis, 1994, 1185–1164 CrossRef CAS; (e) Y. R. Lee and T. V. Hung, Tetrahedron, 2008, 64, 7338–7346 CrossRef CAS; (f) E. Ramesh and R. Raghunathan, Synth. Commun., 2009, 39, 613–625 CrossRef CAS; (g) F. M. Moghaddam, M. Kiamehr, M. R. Khodabakhshi, Z. Mirjafary, S. Fathi and H. Saeidian, Tetrahedron, 2010, 66, 8615–8622 CrossRef CAS; (h) K. C. Majumdar, A. Taher and S. Ponra, Tetrahedron Lett., 2010, 51, 2297–2300 CrossRef CAS.
- E. S. El-Tamany, F. A. El-Shahe and B. H. Mohamed, J. Serb. Chem. Soc., 1999, 64, 9 CAS.
- Z. H. Ismail, G. M. Aly and M. S. El-Degwi, Egyp. J.Biotechnol., 2003, 13, 73 CAS.
- M. E. A. Zaki, H. A. Soliman and A. E. Z. Rashad, Naturforsch. C, 2006, 61, 1 CAS.
- F. M. Abdelrazek, P. Metz, O. Kataeva and A. Jaeger, Arch. Pharm., 2007, 340, 543 CrossRef CAS.
- (a) H. Dürr and H. Bouas-Laurent ed., Photochromism: Molecules and Systems; Elsevier: Amsterdam, 1990 Search PubMed; (b) J. C. Crano and R. J. Guglielmetti ed., Organic Photochromic and Thermochromic Compounds; Kluwer Academic/Plenum Publishers: New York, 1999; Vols. 1 and 2 Search PubMed.
- (a) R. Mannhold, G. Cruciani and H. Weber, J. Med. Chem., 1999, 42, 981 CrossRef CAS; (b) C. W. Brown, S. Liu and D. M. Benbrook, J. Med. Chem., 2004, 47, 1008 CrossRef CAS; (c) B. H. Norman U.S. Patent 0064742 A1, 2008 Search PubMed; (d) C. Conti and N. Desideri, Bioorg. Med. Chem., 2010, 18, 6480 CrossRef CAS.
- (a) R. Mannhold, G. Cruciani and H. Weber, J. Med. Chem., 1999, 42, 981–991 CrossRef CAS; (b) C. W. Brown, S. Liu and D. M. J. Benbrook, J. Med. Chem., 2004, 47, 1008–1017 CrossRef CAS; (c) B. H. Norman, US Pat., 0064742 A1, 2008 Search PubMed; (d) C. Conti and N. Desideri, Bioorg. Med. Chem., 2010, 18, 6480–6488 CrossRef CAS; (e) K. C. Majumdar, A. Taher and S. Ponra, Synthesis, 2010, 4043–4050 CrossRef CAS.
- (a) S. Manikandan, M. Shanmugasundaram and R. Raghunathan, Tetrahedron, 2002, 58, 997–1003 CrossRef; (b) M. J. Khoshkholgh, M. Lotfi, S. Balalaie and F. Rominger, Tetrahedron, 2009, 65, 4228–4234 CrossRef CAS; (c) I. Kim, S. G. Kim, J. Choi and G. H. Lee, Tetrahedron, 2008, 64, 664–671 CrossRef CAS; (d) N. J. Parmar, S. B. Teraiya, R. A. Patel and N. P. Talpada, Tetrahedron Lett., 2011, 52, 2853–2856 CrossRef CAS; (e) T. Kawasaki, M. Nagaoka, T. Satoh, A. Okamoto, R. Ukon and A. Ogawa, Tetrahedron, 2004, 4, 3493–3503 CrossRef; (f) D. Prajapati and M. Gohain, Beilstein J. Org. Chem., 2006, 2, 1 CrossRef.
- (a) K. Tanaka and F. Toda, Chem. Rev., 2000, 100, 1025–1074 CrossRef CAS; (b) V. R. Choudhary, A. Dhar, P. Jana, R. Jha and B. S. Uphade, Green Chem., 2005, 7, 768–776 RSC; (c) N. Deka, A. M. Mariotte and A. Boumendjel, Green Chem., 2001, 3, 263–264 RSC.
- (a) X. L. Li, Y. M. Wang, B. Tian, T. Matsuura and J. B. Meng, J. Heterocycl. Chem., 1998, 35, 129–134 CrossRef CAS; (b) W. Y. Chen and J. Lu, Synlett, 2005, 1337–1339 CrossRef CAS; (c) N. C. Ganguly and M. Datta, Synlett, 2004, 659–662 CrossRef CAS; (d) J. R. Scheffer and K. Wang, Synthesis, 2001, 1253–1257 CrossRef CAS; (e) I. Devi, H. N. Borah and P. J. Bhuyan, Tetrahedron Lett., 2004, 45, 2405–2408 CrossRef CAS; (f) C. J. Mortko, H. Dang, L. M. Campos and M. A. Garibay, Tetrahedron Lett., 2003, 44, 6133–6136 CrossRef CAS.
- A. I. Vogel, Text Book of Practical Organic Chemistry, 5th edition, Longman Group UK Ltd., 2004, pp. 948–949 Search PubMed.
- Y. R. Lee, Y. M. Kim and S. H. Kim, Tetrahedron, 2009, 65, 101–108 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 467–473 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC reference number 820934. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra00930g |
| This journal is © The Royal Society of Chemistry 2012 |
