Cyclodextrin-based dendritic supramolecules; new multivalent nanocarriers†
Mohsen
Adeli
*a,
Mahdieh
Kalantari
a,
Zohreh
Zarnega
a and
Roya
Kabiri
b
aDepartment of Chemistry, Faculty of Science, Lorestan University, Khoramabad, Iran. E-mail: mohadeli@yahoo.com; Fax: (+98) 661-2202782; Tel: (+98) 916-3603772
bLab of NMR, Faculty of Chemistry, Tabriz University, Tabriz, Iran
First published on 29th February 2012
Abstract
Multiarm star copolymers containing a β-cyclodextrin core and poly(2-ethyl-2-oxazoline) arms (CD-POX) were synthesized and characterized. Synthesized CD-POX star copolymers were able to form supramolecular dendritic polymers (SDPs) in aqueous solutions. The ability of SDPs to encapsulate and transfer small guest molecules and also metal nanoparticles was investigated.
A wonderful and well-known case study in the growth of science is the birth of polymer chemistry in the early 20th century.1 With the introduction of supramolecular polymers, which are polymeric arrays of monomeric units brought together by reversible and highly directional noncovalent interactions,2–7 the playground for polymer scientists has broadened and is not limited to covalent polymers only.8 Supramolecular polymers show highly useful properties because they can combine many of the attractive features of conventional polymers with properties that result from reversibility in the bonding under thermodynamic equilibrium.2,3
In recent years, a large number of supramolecular structures prepared by noncovalent interactions such as metal coordinations,9–13 hydrogen bonds14–18 and host–guest19–23 interactions have been reported. When a guest group is covalently attached to a cyclic host, it may form an intramolecular complex or intermolecular complex to give supramolecular polymers.24 One of the main host molecules to form supramolecular systems is cyclodextrin. The geometry of cyclodextrins (CDs) gives a hydrophobic inner cavity and various molecules can be fitted into the cavities to form supramolecular inclusion complexes.25 However, the use of cyclodextrins is limited due to some of their undesirable properties such as low aqueous solubility, particularly beta cyclodextrin (β-CD), or relatively high cost.26 To improve or modify the properties of CDs many modified cyclodextrins, particularly cyclodextrin-containing polymeric systems, have been synthesized.27–29 Combination of polymers with CDs leads to hybrid materials with interesting properties inducted from both the polymers and the CDs.30 For example a linear or dendritic polymer having cyclodextrin in its structure is able to form inclusion complexes with special guest molecules and form supramolecules while it still keep its intrinsic polymeric properties.31,32
Here we describe the preparation and characterization of hybrid materials consisting of a β-cyclodextrin core, poly(2-ethyl-2-oxazoline) arms and diethanolamine or aniline end groups. As will be explained later, dynamic light scattering (DLS), gel permeation chromatography (GPC) and transmission electron microscopy (TEM) studies prove the formation of supramolecular dendritic polymers (SDPs) from CD-POX hybrid materials in aqueous solutions.
As shown in Fig. 1, per-7-iodo-β-cyclodextrin (CD-I) was synthesized by reaction between β-cyclodextrin and iodine in the presence of triphenylphosphine and it was used as a macroinitiator to polymerize 2-ethyl-2-oxazoline monomer through a cationic ring opening polymerization process. In the polymerization process, 2-ethyl-2-oxazoline was added to a solution of CD-I in dried DMF at 140 °C. Afterward the temperature of reaction was reduced to 80 °C and a solution of quencher in dried DMF was injected into the reaction flask and it was stirred for 12 h at the same temperature.
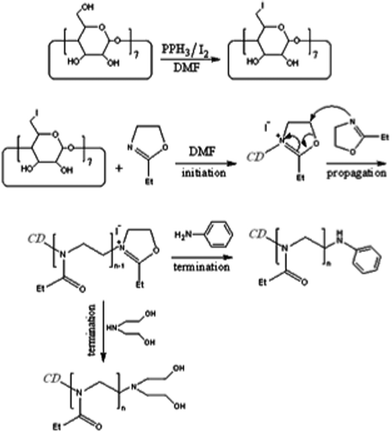 | ||
| Fig. 1 Synthesis of per-7-iodo-β-cyclodextrin (CD-I) and hybrid materials containing poly(2-ethyl-2-oxazoline) chains (POX), β-CD core and aniline or diethanolamine termination groups. | ||
Quenching of the polymerization process by aniline (A) and diethanolamine (D) led to hybrid materials containing aniline (CD-POX-A) and diethanolamine (CD-POX-D) end groups respectively (Fig. 1). The structure of CD-POX-A and CD-POX-D hybrid materials was verified using infrared spectroscopy and 1H-NMR (Electronic Supplementary Information (ESI),† Fig. ESI1 and Fig. ESI2). Differences between the thermal properties of the compounds confirmed that hybrid materials has been synthesised (Fig. ESI3†).
The reason to select aniline and diethanolamine as terminal groups is to investigate the role of cyclodextrin cavities and their host–guest interactions in formation of SDPs, because aniline is a suitable guest molecule for cyclodextrin cavities while diethanolamine can not form inclusion complexes with them.
Different experiments indicated that CD-POX-A and CD-POX-D hybrid materials are able to form SDPs in aqueous solutions. It is believed that the main driving force for self-assembling of CD-POX-A hybrid materials is to form intermolecular inclusion complexes. In general, end aniline moieties of a CD-POX-A hybrid material are included intermolecularly in the cavity of the cyclodextrin of a neighbouring CD-POX-A hybrid material. The proposed structure for self-assembling of CD-POX-A hybrid materials to produce SDPs in aqueous solution is indicated in Fig. 2. As mentioned above, in order to point out whether inclusion complexes between end aniline moieties from one molecule and cyclodextrin from another are the only driving force to produce SDPs, CD-POX-D hybrid material was synthesized and its ability to produce SDPs in aqueous solutions was investigated.
 | ||
| Fig. 2 Schematic representation of (a) CD-POX-A and (b) supramolecular dendritic polymers (SDPs) from CD-POX-A hybrid materials in aqueous solution. | ||
It was found that CD-POX-D hybrid material is also able to form SDPs in aqueous solution. Due to the absence of guest molecules in CD-POX-D hybrid material (the guest molecules in CD-POX-A hybrid material were the end aniline groups), host–guest interactions could not be a driving force to produce SDPs and, in this case, because of the presence of a large number of end hydroxyl functional groups, intermolecular hydrogen bonding is the most probable driving force to produce SDPs. Since noncovalent interactions that are responsible for producing SDPs are a function of concentration, the effect of this parameter on the size and molecular weight of self-assemblies (SDPs) was investigated.
DLS diagrams of CD-POX-A and CD-POX-D hybrid nanomaterials in water at room temperature and at different concentrations are shown in figures ESI4 and ESI5,† respectively. In low concentration (0.5 mg mL−1) solution the diameter of CD-POX-A is much lower than that of CD-POX-D. It is believed that the diameter of CD-POX-A in this concentration is close to that of the molecule itself, because host–guest interactions are the main driving force for assembly of SDPs in aqueous solutions and at this concentration these interactions could not play a critical role. However in the case of CD-POX-D, even in such low concentration the size is big confirming that other noncovalent interactions such as hydrogen bonding dominate formation of SDPs, because it contains a large number of end hydroxyl functional groups. In higher concentrations, the hydrodynamic diameter of CD-POX-A hybrid material increases and it also loses its monomodality (Fig. ESI4c†). The direct relationship between the hydrodynamic diameter of CD-POX-A hybrid material and its concentration in aqueous solution and also the appearance of several different diameters in higher concentrations prove self-assembly and formation of SDPs.
Diagrams in Fig. ESI5† also show the dependence of hydrodynamic diameter of CD-POX-D on the concentration of the solution. These results can be explained simply by secondary supramolecular interactions which lead to dendritic macromolecules with different sizes. It can be found that CD-POX-A and CD-POX-D form large self-assemblies in the aqueous solutions that are stable at room temperature over several months, but the types of forces driving the formation of the assemblies are different. The molecular weight of CD-POX-A was measured by GPC at two concentrations in water as mobile phase. CD-POX-A showed different molecular weights at 0.04 and 0.1 mg mL−1 concentrations (Fig. ESI6†). Since the measured molecular weight of a polymer with covalent bonds is independent of its concentration, this result indicates the formation of a supramolecular dendritic polymer.
We expected that self-assemblies having void spaces would exhibit encapsulation ability. For this reason, we investigated the ability of aqueous solution of CD-POX-A for the encapsulation of palladium nanoparticles (ESI†). For preparation of encapsulated particles, a saturated aqueous solution of PdCl2 was added to 3 mL of an aqueous solution of CD-POX-A (2 mg mL−1) and stirred for 8 h at room temperature. Afterward, a saturated aqueous solution of NaBH4, as reducing agent, was added to above mixture to obtain zerovalent Pd nanoparticles. The mixture was then filtered and the resulting dark brown solution, which was stable for several weeks, was used for TEM experiments. In TEM experiments, a fresh aqueous solution of SDPs containing palladium nanoparticles was dropped on to the surface of a graphitic sampler and the solvent was evaporated at room temperature, then TEM images were recorded. Due to the end hydrophobic aromatic groups (in CD-POX-A), dendritic supramolecules form big collapsed vesicles in water. In these collapsed vesicles the hydrophobic segments turn back to the inside of the assemblies to avoid more interactions with water molecules (Fig. 3a). However TEM images with higher magnification show that each vesicle consists of a large number of dendritic supramolecules having Pd nanoparticles inside (Fig. 3b). There is only one likelihood for the stabilization of Pd nanoparticles by using the aqueous solution of CD-POX-A and that is encapsulation of Pd particles into the cavities of the polymeric system. This is because CD-POX-A alone (a in Fig. 1) does not have a cavity and is not able to encapsulate metal nanoparticles. In contrast, its supramolecular assembly (b in Fig. 1) possesses cavities that metal nanoparticles can be encapsulated into. Fig. 3c shows a TEM image of a supramolecular dendritic polymer in which palladium anchored on the strand-like self-assemblies can be seen.
 | ||
| Fig. 3 TEM images of (a) big vesicles consisting of supramolecular dendritic polymers (scale 5 μ), (b) Pd nanoparticles encapsulated by supramolecular dendritic polymers (scale 500 nm) and (c) a supramolecular dendritic polymer containing palladium nanoparticles placed on strand-like molecular self-assemblies (scale 200 nm). | ||
The availability of the cyclodextrin core of CD-POX-A and CD-POX-D to form inclusion complexes with small guest molecules and therefore the validity of the proposed role for cyclodextrin in the formation of SDPs was tested by UV-vis experiments using ferrocene as a guest molecule. The absorption spectrum of free ferrocene (Fig. ESI8 and Fig.ESI9†) shows two bands with the λmax at about 335 and 450 nm. It can be seen that the intensity of the bands increases upon addition of an aqueous solution of CD-POX-A or CD-POX-D, indicating the formation of inclusion complexes between the β-CD core of both hybrid materials and ferrocene. This experiment shows that the cyclodextrin core of the hybrid materials is available and it can form inclusion complexes with the end aromatic groups of CD-POX-A to create SDPs. However in the case of CD-POX-D although cyclodextrin core is available due to the absence of a suitable end guest molecule (such as aniline in CD-POX-A) it does not play a role in the formation of SDPs. Noncovalent functionalization of carbon nanotubes with various polymers can raise the water solubility of pristine CNTs. This technique was also used to prove the formation of SDPs in aqueous solutions (see ESI†).
In conclusion, our results show that the cavity of star polymers with a cyclodextrin core is available to form inclusion complexes with small guest molecules. Incorporation of small guest molecules on the heads of the arms of star polymers leads to huge dendritic supramolecules in aqueous solutions which are able to encapsulate and transport a variety of nano-objects in their cavities.
References
- M. J. Serpe and S. L. Craig, Langmuir, 2007, 23, 1626 CrossRef CAS.
- L. Brunsveld, B. J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Rev., 2001, 101, 4071 CrossRef CAS.
- A. Harada, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5113 CrossRef CAS.
- E. A. Appel, F. Biedermann, U. Rauwald, S. T. Jones, J. M. Zayed and O. A. Scherman, J. Am. Chem. Soc., 2010, 132, 14251 CrossRef CAS.
- T. Pinault, C. Cannizzo, B. Andrioletti, G. Ducouret, F. Lequeux and L. Bouteiller, Langmuir, 2009, 25, 8404 CrossRef CAS.
- J. Wiergiel, J. Jadyn and L. Bouteiller, J. Phys. Chem. B, 2010, 114, 737 CrossRef.
- H.-J. Kim, T. Kim and M. Lee, Acc. Chem. Res., 2011, 44, 72 CrossRef CAS.
- T. F. A. De Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Rev., 2009, 109, 5687 CrossRef CAS.
- M. Oh, G. B. Carpenter and D. A. Sweigart, Organometallics, 2003, 22, 2364 CrossRef CAS.
- P. Kanoo, K. L. Gurunatha and T. Kumar Maji, Cryst. Growth Des., 2009, 9, 4147 CAS.
- P.-Z. Li, X.-m. Lu, B. Liu, S. Wang and X.-j. Wang, Inorg. Chem., 2007, 46, 5823 CrossRef CAS.
- J.-Y. Lee, C.-Y. Chen, H. M. Lee, E. Passaglia, F. Vizza and W. Oberhauser, Cryst. Growth Des., 2011, 11, 1230 CAS.
- C. D. Nicola, F. Garau, M. Gazzano, M. Monari, L. Pandolfo, C. Pettinari and R. Pettinari, Cryst. Growth Des., 2010, 10, 3120 Search PubMed.
- J. L. Sessler, J. Jayawickramarajah, M. Sathiosatham, C. L. Sherman and S. Brodbelt, Org. Lett., 2003, 5, 2627 CrossRef CAS.
- J.-F. Lutz, A. F. Thünemann and K. Rurack, Macromolecules, 2005, 38, 8124 CrossRef CAS.
- T. Marangoni, S. A. Mezzasalma, A. L. Pallas, K. Yoosaf, N. Armaroli and D. Bonifazi, Langmuir, 2011, 27, 1513 CrossRef CAS.
- A. Bertrand, S. Chen, G. Souharce, C. Ladavire, E. Fleury and J. Bernard, Macromolecules, 2011, 44, 3694 CrossRef CAS.
- R. Schmidt, M. Stolte, M. Grne and F. Wrthner, Macromolecules, 2011, 44, 3766 CrossRef CAS.
- M. Lee, D. V. Schoonover, A. P. Gies, D. M. Hercules and H. W. Gibson, Macromolecules, 2009, 42, 6483 CrossRef CAS.
- L. Zhu, M. Lu, Q. Zhang, D. Qu and H. Tian, Macromolecules, 2011, 44, 4092 CrossRef CAS.
- Z.-X. Zhang, K. L. Liu and J. Li, Macromolecules, 2011, 44, 1182 CrossRef CAS.
- N. L. Strutt, R. S. Forgan, J. M. Spruell, Y. Y. Botros and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 5668 CrossRef CAS.
- T. Ogoshi, Y. Nishida, T.-a. Yamagishi and Y. Nakamoto, Macromolecules, 2010, 43, 3145 CrossRef CAS.
- A. Harada, Y. Kawaguchi and T. Hoshino, J. Inclusion Phenom. Macrocyclic Chem., 2001, 41, 115 CrossRef CAS.
- T. F. A. De Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Soc. Rev., 2010, 39, 495 RSC.
- P. Mura, G. Corti, F. Maestrelli and M. Cirri, J. Inclusion Phenom. Macrocyclic Chem., 2007, 59, 307 CrossRef CAS.
- R. Auzely-Velty, F. Djedaïni-Pilard, S. Desert, B. Perly and Th. Zemb, Langmuir, 2000, 16, 3727 CrossRef CAS.
- O. F. Silva, M. A. Fernandez, S. L. Pennie, R. R. Gil and R. H. d. Rossi, Langmuir, 2008, 24, 3718 CrossRef CAS.
- O. Jazkewitsch, A. Mondrzyk, R. Staffel and H. Ritter, Macromolecules, 2011, 44, 1365 CrossRef CAS.
- S. C. Chan, S. W. Kuo and F. C. Chang, Macromolecules, 2005, 38, 3099 CrossRef CAS.
- M. Adeli, Z. Zarnegar and R. Kabiri, Eur. Polym. J., 2008, 44, 1921 CrossRef CAS.
- A. Zarrabi, M. Adeli, M. Vossoughi and M. A. Shokrgozar, Macromol. Biosci., 2011, 11, 383 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, instruments, experimental procedures and additional references. See DOI: 10.1039/c2ra00813k |
| This journal is © The Royal Society of Chemistry 2012 |
