Shear flow-induced nanotubulation of surface-immobilized liposomes
Yurina
Sekine
ab,
Keita
Abe
a,
Akitaka
Shimizu
a,
Yoshihiro
Sasaki†
*ac,
Shin-ichi
Sawada
d and
Kazunari
Akiyoshi†
*abd
aInstitute of Biomaterials and Bioengineering, Tokyo Medical and Dental University, Kanda-Surugadai, Chiyoda-ku, Tokyo 101-0062, Japan. E-mail: ysasaki.org@tmd.ac.jp
bGlobal Center of Excellence (GCOE) Program, International Research Center for Molecular Science in Tooth and Bone Diseases, Tokyo Medical and Dental University, Japan
cPRESTO, Japan Science and Technology Agency, Japan
dDepartment of Polymer Chemistry, Graduate School of Engineering, Kyoto University, Japan. E-mail: akiyoshi@bio.polym.kyoto-u.ac.jp
First published on 16th February 2012
Abstract
We propose a simple method to prepare lipid nanotubes, which can be used to transport biological molecules. By applying shear stress to surface-immobilized liposomes on a solid substrate, we obtained long lipid nanotubes arranged in a well-controlled direction.
Lipid nanotubes are nanoscale cylindrical objects composed of a lipid bilayer membrane and have attracted much attention in the field of biological science because of their applications in biotechnology and nanotechnology.1 Biological lipid nanotubes, also known as tunneling nanotubes, that connect biological cells over a long distance were recently discovered as a new cell-to-cell communication system.2 The biological nanotubes are formed in a wide variety of cells including neuronal and immune cells to shuttle diverse biochemical cargoes, which include calcium ions and small vesicles. In bionanotechnology, lipid nanotube-based devices with micro- or nanoscale networks have been applied in single-molecule analysis.3
Considering the recent developments and increasing use of lipid nanotubes, there has become a need for protocols that can facilitate their preparation. So far, various techniques to fabricate lipid nanotubes from liposomes have been reported. For example, Orwar et al.4 developed an electroinjection technique that permits the formation of lipid nanotubes and a network between liposomes as reservoirs. Lipid nanotubes can also be formed by self-assembly of tube-forming lipids,1 template-assisted assembly,5 in response to an osmotic pressure difference between the outside and inside of liposomes,6 morphological changes induced by the addition of cholesterol,7 gangliosides,8 or proteins,9 utilization of a microfluidic device,10 and an electric field.11 However, these methods are difficult to handle and offer poor control of the direction and number of lipid nanotubes. The work presented here represents a method to facilitate the formation of lipid nanotubes of up to several millimetres in length by applying shear flow to surface immobilized liposomes (Fig. 1). To the best of our knowledge, this is the first observation of well-aligned and long lipid nanotubes with an internal water phase.
 | ||
| Fig. 1 Experimental setup of the flow chamber (a) and schematic representation of the membrane-bound lipid nanotubes that were formed from surface-immobilized liposomes by applying shear flow (b). | ||
Fluid flow was generated by a closed channeled chamber (μ-Slides VI, ibidi GmbH, Munich, Germany), which can avoid the meniscus and drying effects to apply an homogeneous laminar shear flow within a relatively large area in the flow channel of the chamber. The flow channel (hydrophilic; length, 17 mm; width, 3.8 mm; height, 0.4 mm) was placed between two reservoirs and a reservoir was connected to a computer-controlled syringe pump (REGATO 100, KD Scientific, Holliston, MA, USA) via a luer adaptor and silicon tubes (Fig. 1a). Giant liposomes were immobilized inside this chamber by avidin–biotin interactions (Fig. 1b), essentially as described elsewhere.12 In brief, the flow channel was filled with albumin bovine biotinamidocaproyl (BSA-Biotin) solution (2 mg mL−1 in HEPES buffer, 10 mM, pH 7.4) and incubated for 10 min, followed by washing with HEPES buffer, to provide a biotin-coated channel. This process was repeated twice to completely cover the surface of the chamber with biotin-BSA as previously reported.13 Streptavidin (Type II, 1 mg mL−1) in HEPES buffer was then added to the biotin-coated channel and adsorbed within 40 min. Finally, the channel was washed twice with HEPES buffer. Giant liposomes composed of various molar ratios of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and biotin-modified phospholipid (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[biotinyl(polyethyleneglycol)-2000], DSPE-biotin) were introduced to the flow channel, and left for 10 min to immobilize the liposomes on the inside wall of the channel. The giant liposomes were prepared by gentle hydration of a lipid thin film in a fructose solution14 and the bilayer of the liposome was labeled with a small fraction (0.5 mol%) of a fluorescent lipid probe for fluorescence microscopy (Olympus IX71 epifluorescence microscope equipped with DP70 color CCD camera, Olympus, Japan).
The fluorescence microscopic image (Fig. 2a) clearly showed tubulation when shear flow (300 μL min−1 for 2 min) was applied to the channel immobilizing the liposomes with 1 mol% of DSPE-biotin, whereas some spots of fluorophores indicating liposome without nanotube formation were observed. This flow rate corresponds to a shear stress of 5 μN cm−2. The nanotubes were formed from at least 40% of the liposome (based on the number of liposomes) under current conditions. Fluorescence intensity analysis of the microscopic images showed that the diameters were less than 500 nm and the distribution range was 17%, although it was difficult to determine the exact diameters of the nanotubes from the microscopic image because of the optical limit of the fluorescence microscope. In most cases, extended (downstream) ends of the nanotubes were connected with other liposomes as shown in Fig. 2a. Under the same flow conditions, the liposomes without DSPE-biotin were washed away from the flow channel (data not shown). On the other hand, the liposomal structure was maintained on the wall of the channel when the giant liposomes embedding relatively high concentrations of DSPE-biotin (4 mol%) were immobilized and subjected to the same shear flow (Fig. 2b). This result suggests that strong binding of the liposomes inhibits tubulation. To evaluate the direction of tubulation, the angles between the axis of nanotubes and flow direction (θ) were recorded in five to ten views and the number of nanotubes (n) was plotted against θ for every 1° (Fig. 2c). A strong bias was found in the direction of tubulation, indicating that the nanotubes were formed parallel to the direction of fluid flow.
 | ||
| Fig. 2 Fluorescence microscopic images of lipid nanotubes formed from immobilized DOPC liposomes tethered with 10 μM DSPE-biotin to the flow channel after applying shear flow at 300 μL min−1 (a), and DOPC liposomes (1 mM) immobilized on the chamber with 40 μM DSPE-biotin under the same shear flow (b). The lipid bilayer membranes were labeled with rhodamine DHPE (5 μM). (c) The number of nanotubes was plotted against the angle between the axis of the lipid nanotubes and flow direction (θ) recorded in five to ten views in every 1°. | ||
Tube formation efficiency was quantified by counting the number of tubes passing through a defined area in the flow channel (square area, 1.7 × 1.0 mm). As expected, tubulation was dependent on the concentration of DSPE-biotin tethering the liposomes on the chamber (Fig. 3a). The numbers of lipid nanotubes decreased with increasing concentration of DSPE-biotin up to around 40 μM. Tubulation was greatly reduced at 40 μM DSPE-biotin. Strongly immobilized liposomes in the presence of high concentrations of DSPE-biotin were less likely to be affected by shear flow. The formation of lipid nanotubes from surface-immobilized liposomes was easily controlled by altering the binding intensity of the liposomes onto the flow channel. The flow rate (shear stress) dependence of tubulation was also evaluated using the same experimental setup. As shown in Fig. 3b, the number of nanotubes increases with increasing flow rate to 300 μL min−1. At flow rates exceeding 300 μL min−1, most of the immobilized liposomes disappeared from the flow channel (data not shown). This suggests that very high shear stress either deforms the liposome or washes the liposomes from the chamber.
 | ||
| Fig. 3 Tubulation efficiency was evaluated by counting the number of lipid nanotubes passing through a defined area (1.7 × 1.0 mm) on the flow channel. (a) The number of nanotubes was plotted against the concentration of DSPE-biotin used to tether the liposomes to the chamber with a flow rate of 300 μL min−1. (b) Flow rate dependence of nanotube formation using immobilized liposomes tethered with 10 μM DSPE-biotin. | ||
The presence of an internal water phase provided by lipid bilayer membranes is one of the most important properties of lipid nanotubes for the transportation of water-soluble content. To confirm that the internal phase within the nanotubes was formed by fluid flow, giant liposomes composed of DOPC (1 mM), DSPE-biotin (10 μM), and green fluorescent lipid (N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, NBD-DHPE, 5 μM) incorporating rhodamine B-labeled dextran (Mw = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were prepared. Shear flow was applied to the liposomes immobilized on the flow channel at 300 μL min−1 for 2 min to form the lipid nanotubes. Confocal laser scanning microscopic (FV 10i-DOC, Olympus, Japan) images of nanotubes showed that the red fluorescence of rhodamine B-labeled dextran and green fluorescence of NBD-DHPE overlap (Fig. 4). The nanotubes have a confined space to accommodate at least high molecular weight dextran molecules. The inner spaces of the lipid nanotubes were maintained for at least 5 days without apparent leakage of the fluorescent molecules. This result indicates that the lipid nanotubes were stable during that time. This result indicates the lipid nanotubes have a relatively long life time under the current conditions.
000) were prepared. Shear flow was applied to the liposomes immobilized on the flow channel at 300 μL min−1 for 2 min to form the lipid nanotubes. Confocal laser scanning microscopic (FV 10i-DOC, Olympus, Japan) images of nanotubes showed that the red fluorescence of rhodamine B-labeled dextran and green fluorescence of NBD-DHPE overlap (Fig. 4). The nanotubes have a confined space to accommodate at least high molecular weight dextran molecules. The inner spaces of the lipid nanotubes were maintained for at least 5 days without apparent leakage of the fluorescent molecules. This result indicates that the lipid nanotubes were stable during that time. This result indicates the lipid nanotubes have a relatively long life time under the current conditions.
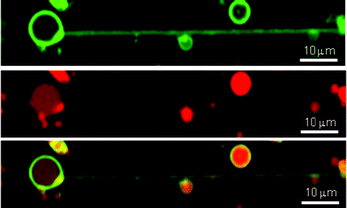 | ||
| Fig. 4 Confocal scanning microscopic images of lipid nanotubes formed from DOPC liposomes. The lipid membrane and internal water phase were stained with NBD-DHPE (0.5 mol%; green) and rhodamine B-labeled dextran (0.1 mM; red), respectively (NBD-DHPE, top; rhodamine B, center; merge, bottom). Bar = 10 μm. | ||
Shear flow-induced transformations of soft materials have been reported in various systems, including stretching of DNA,15 elongation of a spherical liquid drop,16 structural transition of spherical micelles to worm-like micelles17 and transformation of human red blood cells to an ellipsoidal or dumbbell shape.18 Here, we have shown that shear flow induced the formation of lipid nanotubes from liposomes. The mechanical force of the formation of tubular membrane from a giant liposome was reported to be several pN.19 This value is comparable with the force generated by shear flow in this study (several pN for 10 μm liposomes).
In conclusion, we have reported a new method to prepare lipid nanotubes with a controlled direction by applying shear stress to surface-immobilized liposomes. Fluorescence microscopic observation confirmed that the lipid nanotubes have a confined space to accommodate biological molecules. These spaces might be useful as a transport channel or a reaction space of proteins or small molecules. The nanotubulation technique described in this study involves two simple steps, namely liposome immobilization and application of shear flow to the immobilized liposome. Although the present work represents the initial phase of research in shear stress-induced membrane nanotube formation, the simplicity of this system is promising as an efficient and versatile technique to produce lipid nanotubes for use in biotechnology and nanotechnology.
Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research (A) (No. 20240047 to K.A. and Yo.S.) and a Grant-in-Aid for Young Scientists (A) (No. 23681021 to Yo.S.) from the Japan Society for the Promotion of Science (JSPS: “KAKENHI”). This work was supported in part by a Grant-in-Aid for JSPS Fellows (No. 23-5078 to Yu.S.).References
- T. Shimizu, M. Masuda and H. Minamikawa, Chem. Rev., 2005, 105, 1401–1443 CrossRef CAS.
- (a) H. H. Gerdes, N. V. Bukoreshtliev and J. F. Barroso, FEBS Lett., 2007, 581, 2194–2201 CrossRef CAS; (b) D. M. Davis and S. Sownski, Nat. Rev. Mol. Cell Biol., 2008, 9, 431–436 CrossRef CAS; (c) N. M. Sherer and W. Mothes, Trends Cell Biol., 2008, 18, 414–420 CrossRef CAS.
- A. Jesorka and O. Orwar, Annu. Rev. Anal. Chem., 2008, 1, 801–832 CrossRef CAS.
- (a) A. Karlsson, R. Karlsson, M. Karlsson, A.-S. Cans, A. Stroemberg, F. Ryttsen and O. Orwar, Nature, 2001, 409, 150–152 CrossRef CAS; (b) M. Karlsson, K. Sott, M. Davidson, A.-S. Cans, P. Linderholm, D. Chiu and O. Orwar, J. Am. Chem. Soc., 2003, 125, 8442 CrossRef.
- Q. He, Y. Tian, H. Mohwald and J. Li, Soft Matter, 2009, 5, 300–303 RSC.
- H. Hotani, J. Mol. Biol., 1984, 178, 113–120 CrossRef CAS.
- S.-i. M. Nomura, Y. Mizutani, K. Kurita, A. Watanabe and K. Akiyoshi, Biochim. Biophys. Acta, Biomembr., 2005, 1669, 164–169 CrossRef CAS.
- K. Akiyoshi, A. Itaya, S.-i. M. Nomura, N. Ono and K. Yoshikawa, FEBS Lett., 2003, 534, 33–38 CrossRef CAS.
- J. C. Stachowiak, C. C. Hayden and D. Y. Sasaki, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 7781–7786 CrossRef CAS.
- (a) N. Mahajan and J. Fang, Langmuir, 2005, 21, 3153–3157 CrossRef CAS; (b) K. P. Brazhnik, W. N. Vreeland, J. B. Hutchison, R. Kishore, J. Wells, K. Helmerson and L. E. Locascio, Langmuir, 2005, 21, 10814–10817 CrossRef CAS.
- J. A. Castillo, D. M. Narciso and M. A. Hayes, Langmuir, 2009, 25, 391–396 CrossRef CAS.
- M. Gonzalez, L. A. Bagatolli, I. Echabe, J. L. R. Arrondo, C. E. Argarana, C. R. Cantor and G. D. Fidelio, J. Biol. Chem., 1997, 272, 11288–11294 CrossRef CAS.
- T. Ha, Methods, 2001, 25, 78–86 CrossRef CAS.
- S.-i. M. Nomura and K. Akiyoshi, 2006 International Symposium on Micro-Nanomechatronics and Human Science, 2006, 1–6 Search PubMed.
- N. Sonda, M. Hirano, N. Shimada, A. Kano, S. Kidoaki and A. Maruyama, Chem. Lett., 2011, 40, 250–251 CrossRef CAS.
- J. Li, Y. Y. Renardy and M. Renardy, Phys. Fluids, 2000, 12, 269–282 CrossRef CAS.
- T. Shimada, K. Megley, M. Tirrell and A. Hotta, Soft Matter, 2011, 7, 8856–8861 RSC.
- S. P. Sutera and M. H. Mehrjardi, Biophys. J., 1975, 15, 1–10 CrossRef CAS.
- T. Inaba, A. Ishijima, M. Honda, F. Nomura, K. Takiguchi and H. Hotani, J. Mol. Biol., 2005, 348, 325–333 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2012 |
