Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
1H NMR spectroscopic elucidation in solution of the kinetics and thermodynamics of spin crossover for an exceptionally robust Fe2+ complex†
Holm
Petzold
*a,
Paul
Djomgoue
a,
Gerald
Hörner
b,
J. Matthäus
Speck
a,
Tobias
Rüffer
a and
Dieter
Schaarschmidt
a
aTU Chemnitz, Institut für Chemie, Anorganische Chemie, Straße der Nationen 62, 09111 Chemnitz, Germany. E-mail: holm.petzold@chemie.tu-chemnitz.de; Fax: +49-371-53137463
bTU Berlin, Institut für Chemie, Straße des 17. Juni 135, 10623 Berlin, Germany
First published on 27th July 2016
Abstract
A series of Fe2+ spin crossover (SCO) complexes [Fe(5/6)]2+ employing hexadentate ligands (5/6) with cis/trans-1,2-diamino cyclohexanes (4) as central building blocks were synthesised. The ligands were obtained by reductive amination of 4 with 2,2′-bipyridyl-6-carbaldehyde or 1,10-phenanthroline-2-carbaldehyde 3. The chelating effect and the rigid structure of the ligands 5/6 lead to exceptionally robust Fe2+ and Zn2+ complexes conserving their structure even in coordinating solvents like dmso at high temperatures. Their solution behavior was investigated using variable temperature (VT) 1H NMR spectroscopy and VT Vis spectroscopy. SCO behavior was found for all Fe2+ complexes in this series centred around and far above room temperature. For the first time we have demonstrated that the thermodynamics as well as kinetics for SCO can be deduced by using VT 1H NMR spectroscopy. An alternative scheme using a linear correction term C1 to model chemical shifts for Fe2+ SCO complexes is presented. The rate constant for the SCO of [Fe(rac-trans-5)]2+ obtained by VT 1H NMR was validated by Laser Flash Photolysis (LFP), with excellent agreement (1/(kHL + kLH) = 33.7/35.8 ns for NMR/LFP). The solvent dependence of the transition temperature T1/2 and the solvatochromism of complex [Fe(rac-trans-5)]2+ were ascribed to hydrogen bond formation of the secondary amine to the solvent. Enantiomerically pure complexes can be prepared starting with R,R- or S,S-1,2-diaminocyclohexane (R,R-trans-4 or S,S-trans-4). The high robustness of the complexes reduces a possible ligand scrambling and allows preparation of quasiracemic crystals of [Zn(R,R-5)][Fe(S,S-5)](ClO4)4·(CH3CN) composed of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the Zn and Fe complexes with inverse chirality.
1 mixture of the Zn and Fe complexes with inverse chirality.
Introduction
Octahedral complexes of Fe2+ can exist in two different spin states: the paramagnetic high spin (HS) state (S = 2) or the diamagnetic low spin (LS) state (S = 0). In a nitrogen-dominated ligand environment, both spin states are often close in energy and a temperature-dependent equilibrium is found. The molar ratio between the HS state and the LS state can be addressed by external stimuli like irradiation with light, heating or cooling. Such spin crossover (SCO) complexes have been discussed for many applications in, for example, data storage devices or as switches in electronics.1–6 Apart from such sophisticated applications, applications such as thermochromics or in functional magnetic resonance tomography (MRT) can be envisioned.One requirement in this regard is robustness and stability against oxidation and hydrolysis. In particular, the HS Fe2+ complexes are often lacking in robustness due to the small ligand field stabilisation energy. Chelating ligands can help to stabilise HS Fe2+ complexes kinetically as well as thermodynamically.
In this regard, we have recently introduced Fe2+ SCO complexes employing a hexadentate ligand that can be obtained by condensation of 2,2′-diaminobiphenyles and [2,2′-bipyridine]-6-carbaldehyde or 6′-substituted derivatives.7–9 The complexes are stable against hydrolysis in water, but strongly coordinating solvents like dmso replace the hexadentate ligands. We also found that the presence of aminomethylene units directly bound to the aromatic ring increases the sensitivity towards oxidation9,10 leading to imines as well as amides. N-Methylation shuts down this sensitivity but, at the same time, shifts the HS/LS equilibrium completely to the HS state side.9 To circumvent this problem, the 2,2′-diaminobiphenyl bridge can be replaced by aliphatic diamines, e.g., 1,2-diaminocyclohexanes (4). Complexes of this type were reported before by Constable,11 Kol12 and Blackman.13,14 However, all of those Fe2+-complexes were described as LS Fe2+ complexes. Herein, we wish to report topologically related complexes that employ an aliphatic 1,2-diaminocyclohexane (4) fragment as the bridge. These complexes show SCO at elevated temperatures and are exceptionally robust. In addition the rigid structure and robustness of the complexes allow an in-depth survey of the 1H-NMR spectroscopic properties. NMR spectroscopy is one of the most versatile methods to investigate compounds and reactions in solution.15 Here we use this opportunity to present a consistent and general model for the interpretation of 1H NMR spectra of Fe2+ spin crossover complexes in solution. Moreover we will demonstrate that both thermodynamics as well as kinetics of the SCO can be conveniently derived from VT 1H-NMR spectra (VT = variable temperature).
Results and discussion
Synthesis of ligands 5 and 6 and their Fe2+ complexes
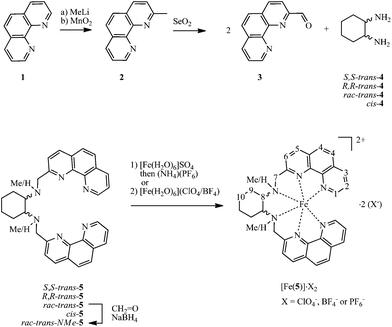
1,10-Phenanthroline-2-aldehyde 3 was prepared following a protocol from Pallavicini et al.16 Starting from 1,10-phenanthroline 1, methylation17 yields compound 2. By oxidation of 2 with SeO2, aldehyde 3 was obtained.16 The ligands 5 were obtained by condensation of 1,10-phenanthroline-2-carbaldehyde 3 with cyclohexane-1,2-diamine 4, followed by reduction with sodium borohydride (Scheme 1). Additional treatment of rac-trans-5 with formaldehyde and sodium borohydride18 allowed the methylation of the secondary amine group to obtain rac-trans-NMe-5. Compounds 5 are in general air sensitive viscous oils.Treatment of ligands 5 with [Fe(OH2)6]SO4 and subsequent anion exchange, or using the appropriate Fe2+ salts, in ethanol or acetonitrile solutions instantly results in the formation of deep purple solutions. The corresponding compounds with the general formula [Fe(5)]X2 precipitate from ethanol solution. The complex salts [Fe(5)]X2 with X = BF4− or ClO4− are well soluble in aprotic, polar organic solvents like acetonitrile (acn), dimethylformamide (dmf), dimethyl sulfoxide (dmso) or acetone. They are sparingly soluble in dichloromethane, ethanol and insoluble in diethyl ether. Exchange of the counter ion with bromide leads to water soluble compounds. Similarly, the analogous bipyridine-based complex [Fe(rac-trans-NMe-6)](BF4)2 was obtained (Scheme 2).
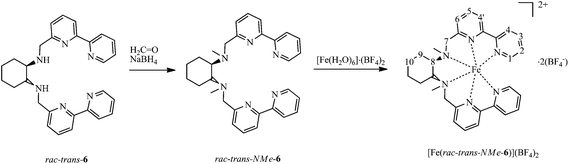 | ||
| Scheme 2 Syntheses of the N-methylated ligand rac-trans-NMe-6 and of compound [Fe(rac-trans-NMe-6)](BF4)2. For synthesis and properties of [Fe(rac-trans-6)](BF4)2, see ref. 11. The numbers in italics give the numbering scheme for discussions on proton NMR spectroscopy. Note that the protons in positions 4/4′ were not unambiguously assigned and therefore treated together. The two chemically different CH2-protons in positions 7, 9, and 10 are referred to as H(7, 9 or 10)-1 and H(7, 9 or 10)-2. | ||
The compounds revealed reasonable elemental analysis data and mass spectra in agreement with mononuclear complexes and a ligand to metal ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It is noted that the NMR spectra of crude [Fe(rac-trans-5)2](BF4)2 showed small additional signals which were assigned to a dinuclear complex. Similar observations were reported by Constable et al. for a related system.11 Materials of significantly higher purity are obtained by recrystallisation from acn/isopropanol. The proton NMR spectra of [Fe(rac-trans-5)2](BF4)2 show typical features of SCO Fe2+ complexes, such as significantly shifted resonances and broad lines. Nevertheless, the expected number of signals requested for a C2 symmetric complex was found in the range of 0.7 to 20 ppm. Proton NMR spectra of the cis-analog [Fe(cis-5)2](BF4)2 are more complicated, likely due to molecular dynamics (vide infra).
1. It is noted that the NMR spectra of crude [Fe(rac-trans-5)2](BF4)2 showed small additional signals which were assigned to a dinuclear complex. Similar observations were reported by Constable et al. for a related system.11 Materials of significantly higher purity are obtained by recrystallisation from acn/isopropanol. The proton NMR spectra of [Fe(rac-trans-5)2](BF4)2 show typical features of SCO Fe2+ complexes, such as significantly shifted resonances and broad lines. Nevertheless, the expected number of signals requested for a C2 symmetric complex was found in the range of 0.7 to 20 ppm. Proton NMR spectra of the cis-analog [Fe(cis-5)2](BF4)2 are more complicated, likely due to molecular dynamics (vide infra).
NMR analysis of spin crossover
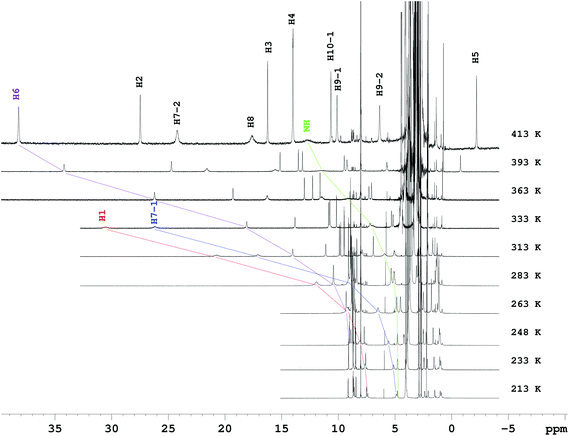 | ||
| Fig. 1 VT 1H NMR spectra of complex [Fe(rac-trans-5)]2+ in d7-dmf-solution. The given temperatures are target temperatures of the probe. | ||
Assignment of the signals to the protons was based on 1H,1H-COSY spectroscopy at low temperature, supplemented by T1 times recorded at room temperature (see below). The assignment of the protons in the HS form of the complexes relies on tracking of the resonances during the transition from the LS to the HS state; a method which benefits from the high transition temperature T½. In cases where this is not possible, we found the analysis of the longitudinal relaxation times (T1 ∝ 1/r6) advantageous, when combined with a proper molecular model. By correlating the paramagnetic contribution to the chemical shift or the linewidths (transversal relaxation time T2) with the H⋯Fe distance, only a rough qualitative picture for the assignment of the proton sites can be obtained. In particular, the chemical shifts are a sum of different contributions (with positive and negative signs) from different coupling mechanisms19 and the linewidths in the spectra of Fe2+ SCO complexes are often dominated by exchange broadening (vide infra).
In order to extract the transition temperature T½ and the thermodynamic parameters ΔSCOH and ΔSCOS of the HS/LS equilibrium, the observed chemical shifts δobs(T) were analysed. Generally, HS/LS equilibration for Fe2+ SCO complexes is rapid with respect to the NMR time scale, so that the chemical shifts are the average of the chemical shifts δHS(T) and δLS of the molecules in the LS and HS states, respectively, weighted by the HS and LS fraction (eqn (1) and (2)). While in eqn (1)–(3), δLS can be treated as temperature independent, the chemical shift of the HS state δHS(T) is intrinsically temperature dependent.20,21 In a first order approximation, the temperature dependence of the chemical shifts in paramagnetic complexes can be calculated using a Curie-like model with a Curie constant Ci(T) for a particular proton site i:20,22δHS(T) = Ci(T)/T + (δdia)i. (δdia)i are the diamagnetic contributions to the chemical shifts of the HS state.
| δobs(T) = γHS(T)δHS(T) + γLS(T)δLS | (1) |
| δobs(T) = γHS(T)δHS(T) + (1 − γHS(T))δLS | (2) |
 | (3) |
 | (4) |
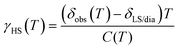 | (5) |
 | (6) |
‘Classical refinement’ with temperature-invariant Curie constants. In most cases studied here, the Curie constants Ci(T) could be satisfyingly treated as temperature independent; that is, Ci(T) = C0i; in the following, this method is named ‘classical refinement’. In addition, δLS and δdia were set equal, subsumed in δdia/LS (justification is given in the ESI†). With this approach, reasonable results had been obtained by others for ΔSCOH and ΔSCOS in related complexes.22,23 In our work, the ‘classical refinement’ was used to calculate ΔSCOH and ΔSCOS for complex [Fe(rac-trans-5)]2+ in d5-pyridine, d7-dmf and d5-nitrobenzene solution, using the six proton sites with the strongest temperature dependence (H1, H7-1, H6, H2, NH, H7-2, H5) (Table 1 gives a summary, details are reported in ESI Tables 3-SI and 4-SI†).
| Solvent ([Fe(rac-trans-5)]2+) | ΔSCOH (kJ mol−1) | ΔSCOS (J mol−1 K−1) | T ½ (K) | max T½ (K) | min T½ (K) |
|---|---|---|---|---|---|
| a Curie constants C0 were set to fixed values, calculated from data of experiments in d7-dmf, d5-nitrobenzene and d5-pyridine solution. b Details for the data processing are reported in the ESI. | |||||
| NMR: full classical refinement | |||||
| d 5-Pyridine | 30.4 | 80 | 381 | 383.1(±3.9); H7-1 | 377.4(±4.8); H6 |
| d 7-dmf | 30.9 | 78.7 | 393 | 396.8(±0.8); H2 | 390.6(±2.1); H5 |
| d 5-nitrobenzene | 28.1 | 79.2 | 355 | 357.1(±0.3); H6 | 352.1(±2.1); H5 |
| NMR: parameterised classical refinementa | |||||
| D2O | 33.7 | 82.9 | 406 | ||
| d 6-dmso | 30.8 | 76.6 | 403 | ||
| d 3-acn | 30.8 | 81.7 | 377 | ||
| VT vis spectroscopyb | |||||
| dmf | 33.4(0.1) | 85.5 | 390.6(0.2) | ||
| nitrobenzene | 28.1(0.2) | 78.3 | 358.4(0.2) | ||
| In epoxy resin | 30.2(0.2) | 79.4 | 380.2(0.2) | ||
In order to allow for fitting to a sigmoidal curve, the product of the HS-fraction and Curie constant was calculated using eqn (6) and (5). Reasonable values for δdia/LS were approximated from spectra recorded at the lowest temperatures accessible. For d5-nitrobenzene with its high melting point, δdia/LS values were approximated by data from d7-dmf solution.
It is found that the freely refined Curie constants Ci(T) differ less than ±10% in different solvents. With the exception of H5, positive Curie constants were found for all protons (for a possible explanation see the ESI†). The two protons H1 and H7-1 with the largest temperature dependence in the chemical shifts yield Curie constants in the range of 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 42
000 and 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm K. The protons at the meta position, with respect to the phenanthroline nitrogen atoms, show smaller Curie constants of about 13
000 ppm K. The protons at the meta position, with respect to the phenanthroline nitrogen atoms, show smaller Curie constants of about 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 20
000 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm K. The NH proton, which is closest to the Fe atom, shows a considerably smaller Curie constant of about 7000 ppm K. These latter results for the nitrogen-bound protons (NH-proton) were rejected as outliers and are only given for completeness.
000 ppm K. The NH proton, which is closest to the Fe atom, shows a considerably smaller Curie constant of about 7000 ppm K. These latter results for the nitrogen-bound protons (NH-proton) were rejected as outliers and are only given for completeness.
An apparently proton-site inherent variance in such-derived transition temperatures T½ of 6.5 K and 5.0 K in d5-pyridine and d5-nitrobenzene, respectively, points to unaccounted secondary temperature effects. Accordingly, more satisfying inner consistency of the transition temperatures was achieved by accounting for the temperature dependence of the Curie constants with 1st-order corrections in T (vide infra). Nevertheless, irrespective of the uncertainties inherent in the classical refinement, [Fe(rac-trans-5)]2+ clearly exhibits solvent-dependent SCO; i.e., the solvent-invoked variance in the transition temperatures (ΔT1/2(solv) ≈ 50 K) significantly exceeds the site-inherent variance (ΔT1/2(site) ≈ 5 K). This finding of a significant solvent effect on the thermodynamics of SCO is of relevance, as only a few examples for solvent-dependent spin crossover have been previously described in the literature. An eminent example was published by Halcrow et al., who associated a variance in T½ of 100 K in water and acetone solution with effects of H-bonding of ligand-appended protic NH groups;24 similar findings had been reported by Linert et al. for complexes with NH-containing benzimidazole ligands,25 corroborating the notion of the cyclohexylamine-borne NH groups harboring the solvent dependence in the present case of [Fe(rac-trans-5)]2+. Related supramolecular effects on the SCO transition temperatures followed from variation of the counterion in nonpolar solvents were reported by Shores.26–28 In agreement with this conclusion, the SCO of the N-methylated congener [Fe(rac-trans-NMe-5)]2+ is found far less susceptible towards solvent effects (vide infra). It is noted that hydrogen bonds are actively influencing SCO in the solid state also and that they hold importance for the occurrence of hysteresis loops in solid samples.29–31
Parameterised ‘classical refinement’ with temperature-invariant Curie constants. In order to put our methodological approach on a broader experimental basis, additional temperature dependent 1H NMR spectra were recorded in d3-acn, d6-dmso and D2O solution. In order to solubilise [Fe(rac-trans-5)](X)2 also in aqueous media, it was extracted from a CH2Cl2/d3-acn solution with D2O in the presence of (NnBu4)(Br).
All three solvents have limitations in the temperature range applicable to solution NMR spectroscopy. This impedes a free refinement of C0; attempts reveal over-parameterisation and unstable refinement. In order to stabilise the refinement and to avoid over-parameterisation, NMR-parameters representing the HS and LS state had to be approximated.
(i) The chemical shifts of proton sites in HS-[Fe(rac-trans-5)]2+ were approximated with fixed Curie constants, Ci. The latter were extracted from previous measurements by averaging the Curie constants in d7-dmf, d5-pyridine and d5-nitrobenzene solution for each particular proton site.
(ii) As an approximation of the chemical shifts of protons in LS [Fe2+(rac-trans-5)]2+, δdia/LS values from d7-dmf solution at low temperature were used.
With these approximations in hand, reasonable fits were obtained (an example for acn solution is given in the ESI, Fig. 9-SI and 10-SI†). The transition temperatures T½ were calculated as 377 K, 403 K and 406 K in d3-acn, d6-dmso and D2O solution, respectively (Table 1). These results corroborate our previous notion of a highly solvent-susceptible SCO in [Fe2+(rac-trans-5)]2+. The second important conclusion to be drawn from these findings is that the compound is exceptionally robust against solvolysis, exposure to moisture, and oxidation in air under harsh conditions, even at elevated temperatures and even in strongly coordinating solvents. The excellent fits with reasonably small ranges of ΔSCOS, the complete reversibility of the phenomena, and the small linewidths at higher temperature are all indicative of the high robustness of the complex. That is, even in the presence of air in dmso solution, NMR spectra could be recorded up to the highest temperature only limited by probe head specifications.
According to our previous results, N-methylation should prevent a degradation of the aminomethylene linker by oxidation. Indeed the N-methylated counterpart [Fe(rac-trans-NMe-5)]2+ is similarly robust. Initial inspection of the temperature dependent 1H NMR spectra clearly revealed a spin equilibrium in [Fe(rac-trans-NMe-5)]2+, compared to the non-methylated congener [Fe(rac-trans-5)]2+, with a higher transition temperature T½. The transition temperatures were calculated by refinement of the observed chemical shifts. Due to the fact that T½ is higher than the highest temperature available for solution 1H NMR spectroscopy, a stable refinement with freely refined Curie constants is not possible. Therefore, fixed Curie constants had to be used, as outlined above for [Fe2+(rac-trans-5)]2+ in the parameterised refinement. As the respective data are not genuinely accessible for [Fe(rac-trans-NMe-5)]2+, we decided to use fixed Curie constants, derived from d7-dmf, d5-pyridine and d5-nitrobenzene solutions of [Fe2+(rac-trans-5)]2+. This data transfer appears justified, because inspection of these spectra revealed an obvious qualitative similarity (direction and magnitude of paramagnetic induced shifts of the resonances) of the NMR spectra and the temperature dependent shifts of [Fe(rac-trans-NMe-5)]2+ and [Fe(rac-trans-5)]2+. It turned out that only for the proton site H7-2 the Curie constants differ notably between the methylated and non-methylated complex. This singularity can be safely assigned to the influence of the additional methyl group (vide infra). Reasonable results of refinement are obtained this way (summary in Table 2, details are in the ESI†), further supporting the validity of the chosen approach.
| [Fe2+(rac-trans-NMe-5)]2+ solution by NMR | ΔSCOH (kJ mol−1) | ΔSCOS (J mol−1 K−1) | T ½ (K) |
|---|---|---|---|
| a Details for the data processing are reported in the ESI. | |||
d
3-acn/d4-acetic acid (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
32.4 | 74.8 | 433 |
| d 6-dmso | 32.5 | 73.6 | 442 |
| d 3-acn | 33.9 | 79.3 | 427 |
| d 5-nitrobenzene | 33.1 | 76.5 | 432 |
| VT UV/Visa | |||
| [Fe2+(rac-trans-NMe-5)]2+ in epoxy resin | 25.8(0.2) | 62.9 | 411.4(0.5) |
| [Fe(cis-5)]2+ acetone solution | 20.1(0.1) | 72.7 | 276.2(0.1) |
In order to validate the results obtained from NMR analysis, we conducted variable temperature Vis-spectroscopy on complex [Fe(rac-trans-5)]2+ in dmf and nitrobenzene solution. The intense MLCT bands between 450 nm and 650 nm are typical of the LS spin state, whereas the HS state absorbs at considerably shorter wavelengths. This allows following the spin crossover by using the absorption at 564 nm. Within experimental errors, the results of the VT Vis spectroscopy and the NMR refinements are consistent (Table 1).
The solution studies, especially in the coordinating solvents like dmso, acetic acid/acn and dmf, were only possible due to the exceptional robustness of the complexes. Robust materials are a prerequisite for many applications. To further demonstrate the inertness of complexes [Fe(rac-trans-5)]2+ and [Fe(rac-trans-NMe-5)]2+, we embedded their hexafluorophosphate salts into an epoxy resin. By simply dissolving the compounds in the hardener and mixing it with the binder we could obtain thermochromic materials. Though the compounds are only sparingly soluble in the hardener, the resulting concentrations (<1 mM) were sufficient to record reversible thermochromic cycles upon heating and cooling. The results from quantitative analyses of the temperature dependent absorption changes at λ = 565 nm agree well with those of the solution studies and confirm SCO to be the molecular origin of the thermochromic effect. It should be noted that the dication [Fe(cis-5)]2+ decomposes during mixing with the hardener and that [Fe(rac-trans-5)]2+ is vulnerable at higher temperature (>400 K) in the presence of oxygen. In contrast, the methylated complex [Fe(rac-trans-NMe-5)]2+ is very robust even in the presence of air.
The derived transition temperatures of [Fe(rac-trans-NMe-5)]2+ fall in a narrow range (427 K < T1/2 < 442 K) with the minimum and maximum values observed in acn and dmso solution, respectively (Table 2). The entropy changes ΔSCOS range from 73.6 J mol−1 K−1 to 79.3 J mol−1 K−1. Given the uncertainties due to the high transition temperatures, the solvent dependence of the spin equilibrium of [Fe(rac-trans-NMe-5)]2+, if any, is not discussed in greater detail. Nevertheless, the transition temperatures of [Fe(rac-trans-NMe-5)]2+ are certainly substantially less prone to solvent effects than [Fe(rac-trans-5)]2+.
The room temperature 1H-NMR spectra for complex [Fe(rac-trans-NMe-6)]2+ show well resolved resonances, typical of a well-defined LS-Fe2+-complex. Not unexpectedly, however, the 1H NMR spectra recorded in d5-nitrobenzene in the temperature range 283 K–403 K (see Fig. 14-SI†), reveal a temperature dependence of the chemical shifts that is akin to [Fe(rac-trans-NMe-5)]2+ and [Fe(rac-trans-5)]2+. However, from these data it is also obvious that the HS fraction of the former complex, even at the highest temperature, is much less than 10% (Fig. 14-SI†). A further reduction in parameters is thus necessary for refinement. An inspection of the entropy data collected in Tables 1 and 2 reveals this parameter to be only weakly susceptible to structure effects, ΔSCOS was fixed at 80 J mol−1 K−1. Using a similar treatment as described before (but with fixed ΔSCOS and Curie constants from d7-dmf solution), the data yielded a reasonable fit for a transition temperature of 570 K. Again obvious deviations are found for the proton H7-2 that is affected by the methyl group.
Attempts to determine the thermodynamic data of the cis-analog complex [Fe(cis-5)]2+ by 1H NMR spectroscopy were unsuccessful, due to atypical behavior (spectra are given in the ESI Fig. 23-SI†). Complementary data obtained by VT Vis spectroscopy indicate an early SCO, being centered at T½ = 276 K and ΔSCOS = 72.7 J mol−1 K−1 (Table 2). The huge effect of stereochemistry of the diamine bridge 4 on the SCO thermodynamics is mainly ascribable to enthalpic effects due to the similarity of ΔSCOS = 72.7 J mol−1 K−1 with the trans-analog complexes. However the principal NMR features, such as decreased paramagnetic shifts at low temperature and the appearance of a new set of broad signals in the typical diamagnetic region (11 ppm to 0 ppm) at 183 K reflect the spin transition deduced from VT Vis spectroscopy. We are not yet able to unambiguously explain these extraordinary broadenings of the NMR resonance lines. Likely additional intramolecular dynamics cause the additional line broadening which hampers analysis of the NMR data; preferential conformations of the cyclohexyl ring are suspected to vary with the spin state.
Improved refinement with 1st-order temperature-dependent Curie constants. It should be noted here that chemical shifts have been measured for complex [Fe(rac-trans-5)]2+ in a temperature range that spans more than 200 K (210 K to 417 K). Although the agreement between calculated and observed chemical shifts was fairly good ((δobs − δcalcd) < 0.3 ppm and ΔγHS(T) < 0.2% see Fig. 5-SI and 8-SI in the ESI†) over the entire temperature range, still there was room and need for improvement. In particular, a look at the confidence intervals calculated for T½ revealed a significant variation in the transition temperatures T½ calculated by using different proton sites for d5-pyridine and d5-nitrobenzene as well as d7-dmf. In other words, from the statistical point of view, the transition temperatures differ significantly with the chosen proton site. This variation is not acceptable, as a refinement using a particular proton site of a given complex must give identical transition temperatures for a particular solvent regardless of the proton site used. This finding points to a small biased error, which we associate with an uncompensated temperature dependence of the Curie constants. Indeed, it has been shown before that both in practice and theory, Ci are temperature dependent.20,32–34 Reasonable schemes taking into account zero field splitting, low lying excited states as well as molecular dynamics have been presented.20,32,33
In the case of Fe2+ complexes, the temperature dependence of C(T) is caused by the successive population of low lying excited states and by zero field splitting.32,35,36 A full treatment of all of these effects in solution NMR spectroscopy is difficult, even in the absence of SCO,33,34 and requires exceptionally accurate NMR data. As a minor point, δdia is not exactly equal to δLS (see Table 2-SI†). In order to account for the temperature dependence of C(T), we decided to follow suggestions from the literature33,37 and applied a linear correction by the introduction of C1 (eqn (7)). The validity of this approach is discussed in detail in the ESI.† As C(T) is given in units of K ppm, C1 must have the dimension of a chemical shift and is given in units of ppm.
| C(T) = C0 + C1T | (7) |
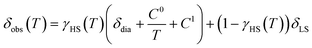 | (8) |
 | (9) |
| C1* = C1 + δdia − δLS | (10) |
As a positive side effect, the new parameter C1 corrects also the difference between δdia and δLS. However, the effects on the results is small and in the following discussion we do not differentiate between C1 and C1* and rename δLS as δdia/LS. In this improved refinement, for every proton site three proton specific parameters, C0, C1, and δdia/LS, need to be refined. A homemade Excel script allowed us to refine only a single global ΔSCOH as well as a single ΔSCOS for all proton sites of a complex together. Necessarily, this approach yields only a single transition temperature irrespective of the number of proton sites used.
To demonstrate the efficiency of this correction, the refinement of 1H NMR data for complex [Fe2+(rac-trans-5)]2+ in d7-dmf, d5-pyridine and d5-nitrobenzene solution was repeated. The global values for ΔSCOH as well as ΔSCOS were set at the averaged values found for a particular solvent in the classical refinement. The proton site specific parameters, C0, C1, and δdia/LS, were refined freely. Very accurate fits were obtained (Fig. 2 and 7-SI†) with very small differences between the calculated and observed chemical shifts (<0.15 ppm) for all resonances at all temperatures and a single ΔSCOH and single ΔSCOS for all proton sites. This demonstrates that the temperature dependence of C(T) is indeed responsible for disagreements in the classical refinement and linear correction with C1 is sufficient to obtain full agreement of calculated and experimentally obtained shifts (Fig. 2). However, we also found that introduction of the parameter C1 also increases the degree of freedom drastically. That is, very accurate fits of the experimental data are obtained at the expense of a wider range of values of ΔSCOH, ΔSCOS and proton specific NMR parameters, again indicating over-parameterisation.
To sum up the NMR approach to the thermodynamics, the correction for the temperature dependency of the Curie constant C(T) according to eqn (7) can be fruitful but only for exceptionally exact data, recorded in large temperature ranges covering temperatures below and considerably above T½. For refinements where the HS fraction remains considerably above 5% at the lowest accessible temperatures due to the high melting point of the solvent, δdia/LS parameters should be fixed at reasonable values. In many cases classical refinement is sufficient. With well-chosen approximations for δdia/LS and/or C0, transition temperatures far above the experimentally available temperature range can be estimated.
A brief description of proton relaxation
Proton spin relaxation in HS-Fe2+ complexes is mainly caused by the dipolar coupling between the proton spin and the spin of the fast relaxing electrons. Hence, the relaxation rates are dependent on the distance of the proton to the metal, the electron relaxation rate (Rs = 1/τs for dipolar relaxation), and the rotational correlation time (τr for Curie relaxation). Solomon–Bloembergen equations (see the ESI†) predict for the proton relaxation rates20 a constant ratio between longitudinal and transversal relaxation (R1 and R2, respectively). For proton relaxation rates, the relationship R2 > R1 must hold. With increasing electron relaxation rates Rs and faster rotation this should approach unity. For octahedral Fe2+ complexes, fast electron relaxation (τs = 10−12 s to 10−13 s)20 is expected. The rotational correlation times τr can be calculated by the Einstein equation using the diameter of the complex and the viscosity of the solvent.Often the assignments of protons of paramagnetic complexes are based on the relationship of the relaxation times Tn (LW frequently named Δν½) to the metal–proton distance r (1/Tn ∝ 1/r6). While the longitudinal relaxation rates (R1 = 1/T1) recorded for [Fe2+(rac-trans-5)]2+ obey this rule perfectly (Fig. 3), the relaxation times (πLW = R2 = 1/T2) recorded around room temperature are not consistent with this rule (see Fig. 4). That is, the relaxation times and, consequently, the linewidths are obviously convoluted with an additional component of dynamics.
The difference between expected and experimental results found for the linewidths can be ascribed to dynamic exchange between the HS and LS state (last term in eqn (11)38). This is corroborated by the large ratio of R2/R1 found for the protons with the largest Curie constants (H1, H7-1) and the strong field dependence of the linewidth, namely of these protons.
 | (11) |
Qualitative description and calculation of kinetics
The first step in the calculation of the kinetics is the determination of the correlation time for the electron relaxation τs. This was achieved using the longitudinal relaxation rates and the distances between the protons and HS Fe2+ ion in [Fe(rac-trans-5)]2+. The latter was approximated with respective Zn–H distances taken from the molecular structure of [Zn(rac-trans-5)]2+, which serves as a structural model. Close structural agreement of iso-ligated complexes of Zn2+ and HS-Fe2+ had been previously found in many systems.39,40 The correlation time for electron relaxation was calculated as τs = 1.1 ps (with 1/τs = 900 GHz ≫ ωI = 3 GHz, as required). The correlation time for the electron relaxation also determines the ratio R2/R1 for the dipolar relaxation, which was calculated as R2/R1 = 1.1. With the calculated additional contribution of Curie relaxation to R2, the expected ratio is R2/R1 = 1.46. With the known ratio R2/R1, the contribution of the exchange between HS and LS species to the linewidths can be isolated using eqn (11). The effect of H,H-coupling was corrected by subtraction.We found that for protons H1 and H7-1, the linewidth is largely dominated by the exchange broadening. This observation can be ascribed to the large Curie constants (>30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm K with a large difference in the chemical shift between the HS and LS state) and, secondly, to the rather large distance of the proton site from the Fe2+ ion (>3.3 Å with large T1). Both facts make these protons the best choice for further analysis (further useful sites are H6 and H2). The NH proton and H8 appear not to be suitable, being both close to the metal centre with large longitudinal relaxivity R1 and a comparatively small Curie constant.
000 ppm K with a large difference in the chemical shift between the HS and LS state) and, secondly, to the rather large distance of the proton site from the Fe2+ ion (>3.3 Å with large T1). Both facts make these protons the best choice for further analysis (further useful sites are H6 and H2). The NH proton and H8 appear not to be suitable, being both close to the metal centre with large longitudinal relaxivity R1 and a comparatively small Curie constant.
Based on H1, H2, H6 and H7-1, the time constant for the exchange between HS and LS state τex (1/τex = kHL + kLH) was calculated to be 44 ns, 33 ns and about 28 ns at 293 K, 300.3 K and 316.1 K, respectively (Tables 12-SI to 14-SI†). To test the inner consistency of the calculations, the linewidth at 293 K for protons H1, H2, H6 and H7-1 as well as NH, H8, H5 and H7-2 at half the magnetic field was calculated and they agree well with experimentally obtained spectra (ESI Table 13-SI and Fig. 22-SI†).
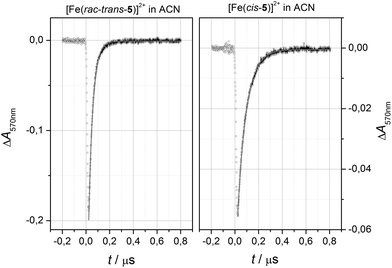
The NMR-derived results for the exchange kinetics (τex) of [Fe(rac-trans-5)]2+ were validated by laser flash photolysis (LFP) experiments in acn solution at ambient temperature. This method relies on light-induced population of the HS state via a rapid and selective relaxation cascade from the initially populated MLCT state. Decay of transient absorption directly reports on the kinetics of re-equilibration; mono-exponential fits to the experimental data provide reliable reference data for the relaxation kinetics of SCO compounds.41–46
Representative decay profiles obtained after excitation of the LS components of [Fe(rac-trans-5)]2+ and [Fe(cis-5)]2+ at λexc = 532 nm in dilute acn solution are shown in Fig. 5. The decay profiles have been recorded at the maximum wavelength of the intense MLCT transitions in the visible spectra range. The appearance as a bleach signal corresponds to the transient population of the spectroscopically transparent HS state. As required for uni-molecular reactions, the decay of the bleach signals is convincingly modeled by mono-exponential decay functions. The lifetimes of signal decay τex are extracted from the fits. They closely agree with the NMR-derived values (τex(1H-NMR) = 33.7 ns vs. τex(LFP/acn) = 35.8(0.16) ns). We take this compliance of methods as good evidence in favor of the herein applied NMR-based access to SCO kinetics. The individual reaction rates for the forward and backward reaction calculate to kHL = 27.5 MHz and kLH = 2.2 MHz, respectively. For sake of comparison, the relaxation rates of [Fe(cis-5)]2+ are also extracted. We find them to be significantly longer (τex(ACN) = 75.2(0.3) ns). This divergence reflects the lower thermodynamic driving force which is represented by the lower transition point T1/2 of the latter compound.
Crystal structures
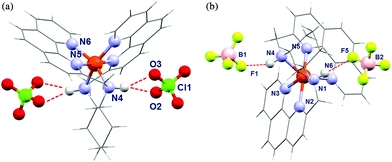
The molecular structures of complex dications [Zn(rac-trans-5)]2+, [Fe(rac-trans-NMe-5)]2+, [Fe(cis-5)]2+ and [Fe(rac-trans-NMe-6)]2+ were investigated by X-ray structure analysis in the solid state (Tables 16-SI and 167-SI†). All complexes are mononuclear with a distorted octahedral coordination sphere (Fig. 6 and 25-SI†). As expected the hexadentate ligands wrap around the Fe2+ ion with their two arms (one including N1–N2–N3 and the other N4–N5–N6). The two arms complement in a meridional arrangement similar to the situation in [Fe(terpy)2]2+ (terpy = 2,2′:6′,2′′-terpyridine)47–49 and related complexes.7,9–11,13,14The secondary amines in [Zn(rac-trans-5)]2+ and [Fe(cis-5)]2+ form hydrogen bonds with the counter ions (BF4− and ClO4−). This finding supports the notion that hydrogen bonds are indeed responsible for the solvent dependence of T½ observed for [Fe(rac-trans-5)]2+. The diffraction data were collected at temperatures below 160 K. The high transition temperatures T1/2 found in solution for the trans-complexes, transcribe into primarily LS character below room temperature. Accordingly, the Fe2+ ions in the crystal structures of [Fe(rac-trans-NMe-5)]2+ and [Fe(6)]2+ were found in the low spin state, as evidenced by the Fe–N bond lengths around 2.0 Å, both to the amine nitrogen atoms N1/N4 and to the terminal pyridine nitrogen atoms N3/N6. The Fe–N bond length to the inner pyridine rings, akin to [Fe(terpy)2]2+,47–50 is even shorter (1.88 Å).
Bond lengths of the Fe2+ ion to the donating nitrogen atoms are much longer in [Fe(cis-5)]2+ (2.23 Å to secondary amine and terminal pyridine nitrogens versus 2.1 Å to the inner pyridine) and are indicative of the HS state. The cyclohexyl ring of [Fe(cis-5)]2+ is found in a preferred chair conformation. However, close intramolecular contact between two CH2 units (C⋯C distance of 2.953 Å between C(H7)2 and C(H9)2) points to repulsions between the amino methylene group and the cyclohexyl ring that might destabilise the chair conformation and favor a twist boat conformer in the LS state. The activation energy of the transition of the chair conformer to the next stable twist boat conformer (20 kJ mol−1 above the chair conformation) is 44 kJ mol−1.51,52 This falls well within the available energy range to result in the observed irregular line broadening of this compound. A fast and complete ring flip at the NMR time scale would result in racemisation and in a pseudo C2-symmetry in the NMR spectra; however no indication of higher symmetry was found in the NMR spectra.
Unfortunately any attempt to obtain [Fe(rac-trans-5)]X2 as a single-crystal suitable for X-ray structure analysis failed. Irrespective of the chosen counter ions X−, only microcrystalline samples were obtained. In contrast, the salt [Zn(rac-trans-5)](ClO4)2 crystallises in well-shaped crystals suitable for X-ray analysis. To obtain the molecular structure of [Fe(rac-trans-5)]2+ (and to demonstrate its stability against ligand scrambling), we prepared the two enantiomerically pure complexes [Zn(R,R-5)](ClO4)2 and [Fe(S,S-5)](ClO4)2 starting from commercially available enantiomerically pure diamines R,R-trans-4 and S,S-trans-4. Both complexes were combined as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture in acetonitrile. To our delight, slow diffusion of ether vapors to this solution yielded crystals suitable for single crystal structure analysis.
1 mixture in acetonitrile. To our delight, slow diffusion of ether vapors to this solution yielded crystals suitable for single crystal structure analysis.
Refinement in I2 leads to two symmetry independent cations [M(R,R-trans-5)]2+ and [M(S,S-trans-5)] with clearly different M–N bond lengths. One set of bond lengths is typical of LS Fe2+ while the other is typical of Zn2+, allowing a preliminary assignment. In agreement with this assignment, the chiral configurations of the molecules matched the configurations of the starting materials and verified the quasi racemic crystal structure.12 To verify the results, ESI mass spectra were recorded using the original crystal that had been used for data collection. Indeed, both cations were found in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with the expected isotopic pattern (Fig. 26-SI†), thus corroborating the assignment of the crystal composition as [Zn(R,R-5)][Fe(S,S-5)](ClO4)4·(CH3CN). In the crystal structure, the [Zn(R,R-5)]2+ cations and [Fe(S,S-5)]2+ cations are threaded along different C2 axis with an opposite sense of direction. As a consequence, both cations have crystallographic induced C2 symmetry. Between the phenanthroline groups of the Zn and Fe complex cations π–π contacts (3.3 Å plane distance) were found, forming a chain with alternation of Fe and Zn cations along the direction of the a-axis (Fig. 7).
1 ratio with the expected isotopic pattern (Fig. 26-SI†), thus corroborating the assignment of the crystal composition as [Zn(R,R-5)][Fe(S,S-5)](ClO4)4·(CH3CN). In the crystal structure, the [Zn(R,R-5)]2+ cations and [Fe(S,S-5)]2+ cations are threaded along different C2 axis with an opposite sense of direction. As a consequence, both cations have crystallographic induced C2 symmetry. Between the phenanthroline groups of the Zn and Fe complex cations π–π contacts (3.3 Å plane distance) were found, forming a chain with alternation of Fe and Zn cations along the direction of the a-axis (Fig. 7).
Electrochemistry
The previous discussion of the 1H NMR and UV/Vis spectra pointed out effects of N-methylation of the ligand-appended NH groups on the thermodynamics of SCO. Cyclic voltammetry was used to track concomitant effects on the redox chemistry (Table 18-SI and Fig. 28-SI†). All complexes under study show reversible or quasi-reversible oxidation processes. Exceptionally, the redox wave of [Fe(rac-trans-NMe-6)]2+/3+ has a large peak separation of 103 mV. The large peak separation likely occurs for the same reason as previously given for the unusual NMR broadening; we think that the redox event includes two redox couples with slightly different redox potentials for two different conformations of the cyclohexyl ring. An alternative interpretation in terms of SCO appears unlikely, because for Fe2+ SCO complexes usually only one well reversible redox process is found due to the fast spin equilibrium.53A substantial effect of N-methylation on the metrics of both, SCO and redox, became evident in previous work on a structurally related complex, employing a 2,2′-diamino-1,1′-biphenyl bridge.9 For instance, in this latter case, the methylation of the secondary amine resulted in a dramatic shift of the spin-state energies. Thermal SCO behavior applied to the non-N-methylated complex (T½ = 403 K), whereas purely HS character arises for the N-methylated complex. At the same time, the oxidation potential for the Fe2+/3+ redox transition shifted by 300 mV to more anodic potentials.9 Both effects were ascribed to steric reasons; that is, to the repulsion between the methyl groups and the biphenyl core that hinders the approach of the ligand to the metal center and therefore favors the HS state or the Fe2+/3+-ion with the larger ion diameter.
Also in the present case the N-methylation has a significant impact on the redox potentials. Correspondingly, the complexes investigated in this study can be grouped into two categories along the status of substitution of the amine groups: accordingly and in agreement with previous experience, non-N-methylated complexes (E0′ = 465 mV ([Fe(rac-trans-5)]2+/3+); E0′ = 487 mV ([Fe(cis-5)]2+/3+)) are more susceptible towards oxidation than their N-methylated congeners (E0′ = 570 mV [Fe(rac-trans-NMe-5)]2+/3+ and E0′ = 540 mV [Fe(rac-trans-NMe-6)]2+/3+). Akin to the aforementioned 2,2′-diamino-1,1′-biphenyl bridged complexes, N-methylation causes an anodic shift of the redox potentials, admittedly by only 100 mV. However, here the stabilization of the Fe2+-redox state by N-methylation is accompanied by an inverted effect on T½. That is, for the two redox pairs [Fe(rac-trans-5)]2+/3+ and [Fe(rac-trans-NMe-5)]2+/3+, the methylation stabilises the LS state.
On first glance this seems contradictory; the N-methylated tertiary amine is a better donor than the secondary amine and should support the higher oxidation state, but an opposite effect is observed. Likely, the reason is the increased shielding of the metal center by the methyl groups. Charges are usually screened by a surrounding solvent cage. The dipole moments of the oriented solvent molecules are preferentially aligned in an opposite direction with the electric field and reduce the effective charge on the metal complex. The non-methylated complexes [Fe(rac-trans-5)]2+/3+ and [Fe(cis-5)]2+/3+ can form hydrogen bonds that support the alignment of solvent (acn) dipole moments opposite to the electric field and therefore increase the charge compensating effect of the solvent cage. This effect facilitates the oxidation of non-N-methylated complexes and stabilises the N-methylated complexes in the Fe2+ oxidation state. Obviously this effect is larger than the electron donating effect of the additional methyl groups [Fe(rac-trans-NMe-5)]2+/3+. This line of argumentation is in agreement with the outcomes of the solvatochromism studies (see the ESI†).
Conclusion
In conclusion, we have prepared a series of Fe2+ SCO complexes employing hexadentate ligands with 1,2-diaminocyclohexanes (4) as central building blocks. The chelating effect and the rigid structure of the ligand make the complexes exceptionally robust. They are inert even against coordinating solvents like dmso at high temperatures. We investigated their solution behaviour by using VT 1H NMR spectroscopy and VT Vis spectroscopy. For the first time we have demonstrated that by using VT 1H NMR spectroscopy the thermodynamics as well as kinetics for SCO can be obtained. VT 1H NMR spectroscopy has the major advantages of being a species-specific, non-bulk method offering the high precision of a few Hz in a range of about 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz. The complex investigated in this survey, namely [Fe(rac-trans-5)]2+, shows a comparatively fast SCO with correlation time in the 10 ns-timescale. With increasing correlation time for SCO (τsco = 1/kex), the ratio between exchange broadening and paramagnetic relaxation becomes more convenient for the analysis and allows instantaneous identification of slow SCO processes in Fe2+–SCO-complexes.41,54 Moreover, use of very high field magnets (>600 MHz) will shorten the timescale further allowing elucidation of SCO kinetics down to the 1 ns-timescale. The solvent dependence of T1/2 found for complex [Fe(rac-trans-5)]2+ is ascribed to hydrogen bond formation of the secondary amine and the solvent. The cyclic voltammetry experiments also revealed the dominant effect of the hydrogen bonds to the secondary amine on the redox potentials. Enantiomeric pure complexes can be prepared starting with R,R- or S,S-1,2-diaminocyclohexane (R,R-trans-4 or S,S-trans-4). The high robustness of the complexes reduces a possible ligand scrambling and allows preparation of quasiracemic crystals of [Zn(R,R-5)][Fe(S,S-5)](ClO4)4·(CH3CN) including a 1
000 Hz. The complex investigated in this survey, namely [Fe(rac-trans-5)]2+, shows a comparatively fast SCO with correlation time in the 10 ns-timescale. With increasing correlation time for SCO (τsco = 1/kex), the ratio between exchange broadening and paramagnetic relaxation becomes more convenient for the analysis and allows instantaneous identification of slow SCO processes in Fe2+–SCO-complexes.41,54 Moreover, use of very high field magnets (>600 MHz) will shorten the timescale further allowing elucidation of SCO kinetics down to the 1 ns-timescale. The solvent dependence of T1/2 found for complex [Fe(rac-trans-5)]2+ is ascribed to hydrogen bond formation of the secondary amine and the solvent. The cyclic voltammetry experiments also revealed the dominant effect of the hydrogen bonds to the secondary amine on the redox potentials. Enantiomeric pure complexes can be prepared starting with R,R- or S,S-1,2-diaminocyclohexane (R,R-trans-4 or S,S-trans-4). The high robustness of the complexes reduces a possible ligand scrambling and allows preparation of quasiracemic crystals of [Zn(R,R-5)][Fe(S,S-5)](ClO4)4·(CH3CN) including a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the Zn and Fe complexes with inverse chirality. Furthermore we demonstrated the incorporation of the complexes [Fe(rac-trans-5)]2+ and [Fe(rac-trans-NMe-5)] in epoxy resins by dissolving an appropriate salt in the hardener before mixing with the resin.
1 mixture of the Zn and Fe complexes with inverse chirality. Furthermore we demonstrated the incorporation of the complexes [Fe(rac-trans-5)]2+ and [Fe(rac-trans-NMe-5)] in epoxy resins by dissolving an appropriate salt in the hardener before mixing with the resin.
Experimental
Experimental details for the synthesis and characterisation of the materials, details on the crystal structure refinement and a detailed description of the methods used are compiled in the ESI.† The results of the crystal structure analysis for compounds [Fe(cis-5)](BF4)2·(0.5·CH3CN) (CCDC 1472618), [Fe(rac-trans-NMe-5)](BF4)2·(0.5·CH3CN) (1472619), [Zn(rac-trans-5)](ClO4)2·(CH3CN) (1472620), [Zn(R,R-5)][Fe(S,S-5)](ClO4)4·2(CH3CN) (1472621) and [Fe(rac-trans-NMe-6)](BF4)2 (1472622) were deposited in the CSD data base.Acknowledgements
This work was financially supported by the DAAD, Fonds der Chemischen Industrie (Liebig Stipendium) and the Deutsche Forschungsgemeinschaft DFG (PE1513/4-1). G. H. thanks Dr Tomasz Pedzinski (AMU Poznan) for assistance with laser-flash photolysis. G. H. acknowledges support of this work by the Deutsche Forschungsgemeinschaft(SFB 658, Elementary processes in molecular switches on surfaces).Notes and references
- M. A. Halcrow, Structure: Function Relationships in Molecular Spin-Crossover Materials, Wiley & Sons, Chichester, 2013 Search PubMed.
- P. Gütlich, Y. Garcia, H. A. Goodwin, Y. Garcia and H. A. Goodwin, Chem. Soc. Rev., 2000, 29, 419–427 RSC.
- P. Gütlich and A. Hauser, Coord. Chem. Rev., 1990, 97, 1–22 CrossRef.
- H. Toftlund, Coord. Chem. Rev., 1989, 94, 67–108 CrossRef CAS.
- P. Gütlich, A. B. Gaspar and Y. Garcia, Beilstein J. Org. Chem., 2013, 9, 342–391 CrossRef PubMed.
- P. Gütlich, A. Hauser and H. Spiering, Angew. Chem., Int. Ed., 1994, 106, 2109–2141 Search PubMed.
- H. Petzold and S. Heider, Eur. J. Inorg. Chem., 2011, 1249–1254 CrossRef CAS.
- S. Heider, H. Petzold, G. Chastanet, S. Schlamp, T. Rüffer, B. Weber and J.-F. Létard, Dalton Trans., 2013, 42, 8575–8584 RSC.
- S. Heider, H. Petzold, J. M. Speck, T. Rüffer and D. Schaarschmidt, Z. Anorg. Allg. Chem., 2014, 640, 1360–1367 CrossRef CAS.
- S. Heider, H. Petzold and G. Teucher, Eur. J. Inorg. Chem., 2013, 2013, 2382–2388 CrossRef CAS.
- E. C. Constable, G. Zhang, C. E. Housecroft, M. Neuburger and J. A. Zampese, Eur. J. Inorg. Chem., 2010, 2000–2011 CrossRef CAS.
- Y. Popowski, I. Goldberg and M. Kol, Chem. – Eur. J., 2016, 22, 5530–5533 CrossRef CAS PubMed.
- N. A. Hall, C. Duboc, M.-N. Collomb, A. Deronzier and A. G. Blackman, Dalton Trans., 2011, 40, 12075–12082 RSC.
- L. J. Baird, C. A. Black and A. J. Blackman, Polyhedron, 2007, 26, 378–384 CrossRef CAS.
- M. P. Shores, C. M. Klug and S. R. Fiedler, in Spin-Crossover Mater, ed. M. A. Halcrow, Wiley & Sons, 2013, pp. 281–301 Search PubMed.
- P. Pallavicini, V. Amendola, Y. D. Fernandez, M. Ghisalberti, L. Linati, C. Mangano, A. M. Lanfredi and C. Massera, Dalton Trans., 2003, 575–580 RSC.
- V. Hebbe-Viton, V. Desvergnes, J. J. Jodry, C. Dietrich-Buchecker, J.-P. Sauvage and J. Lacour, Dalton Trans., 2006, 2058–2065 RSC.
- G. J. P. Britovsek, J. England and A. J. P. White, Dalton Trans., 2006, 1399–1408 RSC.
- A. Borgogno, F. Rastrelli and A. Bagno, Dalton Trans., 2014, 43, 9486 RSC.
- G. P. I. Bertini and C. Luchinat, Solution NMR of Paramagnetic Molecules, Elsevier, 2001 Search PubMed.
- J. P. Jesson, S. Trofimenko and D. R. Eaton, J. Am. Chem. Soc., 1967, 89, 3158–3164 CrossRef CAS.
- K. Bryliakov, E. Duban and E. Talsi, Eur. J. Inorg. Chem., 2005, 72–76 CrossRef CAS.
- S. E. Creutz and J. C. Peters, Inorg. Chem., 2016, 55, 3894–3906 CrossRef CAS PubMed.
- S. A. Barrett, C. A. Kilner and M. A. Halcrow, Dalton Trans., 2011, 40, 12021–12024 RSC.
- B. Strauß, V. Gutmann, W. Linert and B. Strauß, Monatsh. Chem., 1993, 124, 515–522 CrossRef.
- Z. Ni, A. M. McDaniel and M. P. Shores, Chem. Sci., 2010, 1, 615 RSC.
- Z. Ni and M. P. Shores, J. Am. Chem. Soc., 2009, 131, 32–33 CrossRef CAS PubMed.
- Z. Ni and M. P. Shores, Inorg. Chem., 2010, 49, 10727–10735 CrossRef CAS PubMed.
- B. Weber, W. Bauer, T. Pfaffeneder, M. M. Dîrtu, A. D. Naik, A. Rotaru and Y. Garcia, Eur. J. Inorg. Chem., 2011, 2011, 3193–3206 CrossRef CAS.
- B. Weber, E. S. Kaps, J. Obel, K. Achterhold and F. G. Parak, Inorg. Chem., 2008, 47, 10779–10787 CrossRef CAS PubMed.
- B. Weber, W. Bauer and J. Obel, Angew. Chem., 2008, 120, 10252–10255 CrossRef.
- B. Weber and F. A. Walker, Inorg. Chem., 2007, 46, 6794–6803 CrossRef CAS PubMed.
- P. Basu, N. V. Shokhirev, J. H. Enemark and F. A. Walker, J. Am. Chem. Soc., 1995, 117, 9042–9055 CrossRef CAS.
- F. A. Walker, Inorg. Chem., 2003, 42, 4526–4544 CrossRef CAS PubMed.
- B. Weber, J. Obel, D. Henner-Vasquez and W. Bauer, Eur. J. Inorg. Chem., 2009, 5527–5534 CrossRef CAS.
- B. Weber, C. Carbonera, C. Desplances and J.-F. Letard, Eur. J. Inorg. Chem., 2008, 1589–1598 CrossRef CAS.
- Z. Xia, B. D. Nguyen and G. N. La Mar, J. Biomol. NMR, 2000, 17, 167–174 CrossRef CAS PubMed.
- J. S. Leigh, J. Magn. Reson., 1971, 311, 308–311 Search PubMed.
- N. Paradis, G. Chastanet, F. Varret and J. Létard, Eur. J. Inorg. Chem., 2013, 2013, 968–974 CrossRef CAS.
- P. Chakraborty, C. Enachescu, C. Walder, R. Bronisz and A. Hauser, Inorg. Chem., 2012, 51, 9714–9722 CrossRef CAS PubMed.
- P. Stock, T. Pędziński, N. Spintig, A. Grohmann and G. Hörner, Chem. – Eur. J., 2013, 19, 839–842 CrossRef CAS PubMed.
- S. Schenker, P. C. Stein, J. A. Wolny, C. Brady, J. J. Mcgarvey, H. Toftlund and A. Hauser, Inorg. Chem., 2001, 40, 134–139 CrossRef CAS PubMed.
- J. J. McGarvey, H. Toftlund, A. H. R. Al-Obaidi, K. P. Taylor and S. E. J. Bell, Inorg. Chem., 1993, 32, 2469–2472 CrossRef CAS.
- A. H. R. Al-Obaidi, K. B. Jensen, J. J. McGarvey, H. Toftlund, B. Jensen, S. E. J. Bell and J. G. Carroll, Inorg. Chem., 1996, 35, 5055–5060 CrossRef CAS PubMed.
- P. Stock, E. Deck, S. Hohnstein, J. Korzekwa, K. Meyer, F. W. Heinemann, F. Breher and G. Hörner, Inorg. Chem., 2016, 55, 5254–5265 CrossRef CAS PubMed.
- P. Stock, N. Spintig, J. Scholz, J. D. Epping, C. Oelsner, D. Wiedemann, A. Grohmann and G. Hörner, J. Coord. Chem., 2015, 68, 3099–3115 CrossRef CAS.
- A. Hauser, C. Enachescu, M. L. Daku, A. Vargas and N. Amstutz, Coord. Chem. Rev., 2006, 250, 1642–1652 CrossRef CAS.
- F. Pelascini, M. Wesolek, F. Peruch, A. De Cian, N. Kyritsakas, P. J. Lutz and J. Kress, Polyhedron, 2004, 23, 3193–3199 CrossRef CAS.
- G. U. Priimov, P. Moore, P. K. Maritim, P. K. Butalanyi and N. W. Alcock, J. Chem. Soc., Dalton Trans., 2000, 1, 445–449 RSC.
- L. J. K. Cook, F. Tuna and M. A. Halcrow, Dalton Trans., 2013, 42, 2254–2265 RSC.
- D. Bucher, L. C. T. Pierce, J. A. McCammon and P. R. L. Markwick, J. Chem. Theory Comput., 2011, 7, 890–897 CrossRef CAS PubMed.
- G. Gill, D. M. Pawar and E. A. Noe, J. Org. Chem., 2005, 10726–10731 CrossRef CAS PubMed.
- J. W. Turner and F. A. Schultz, Coord. Chem. Rev., 2001, 219–221, 81–97 CrossRef CAS.
- E. C. Constable, G. Baum, E. Bill, R. Dyson, R. V. Eldik, D. Fenske, S. Kaderli, D. Morris, A. Neubrand and M. Neuburger, et al. , Chem. – Eur. J., 1999, 5, 498–508 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1472618–1472622. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt01895e |
| This journal is © The Royal Society of Chemistry 2016 |