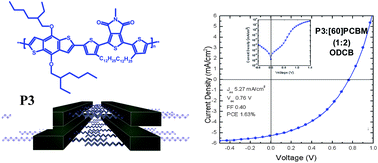
Four new donor–acceptor semiconducting alternating copolymers consisting of thienopyrroledione (TPD) electron-accepting sub-units and either 2,7-carbazole or dialkoxy substituted benzodithiophene electron-donating moieties were prepared either by Suzuki or Stille coupling. Analytical size exclusion chromatography (SEC) was used to fractionate the copolymer with 1 : 1 thienopyrroledione to carbazole ratio into 9 sharp fractions. The effective conjugation length (35 aromatic rings) was determined on the basis of the dependence of λmax of the π–π* band on the degree of polymerization, DPn. All four polymers showed similar supramolecular organization resembling that of poly(3-alkylthiophene)s since the mechanism of the self-assembly was the same in both cases: π-stacking of the π-conjugated polymer backbones and interdigitation of n-alkyl side chains. As shown by cyclic voltammetry studies, three copolymers showed band gaps inferior to 2 eV with HOMO and LUMO levels ranging between −5.62 eV to −5.08 eV and −3.53 eV to −3.13 eV, respectively. UV-vis-NIR spectroelectrochemical investigations confirmed the results obtained by cyclic voltammetry, enabling in addition more precise determination of the HOMO level. Raman spectroelectrochemical studies showed that the polymer with 1 : 1 thienopyrroledione to carbazole ratio is prone to oxidative degradation, consistent with cyclic voltammetry studies. A bulk-heterojunction solar cell was fabricated from the copolymer consisting of thienopyrroledione and dialkoxy substituted benzodithiophene. A power conversion efficiency of 1.63% was achieved for non-optimized devices.