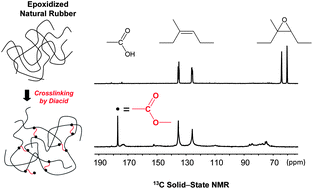
The crosslinking of epoxidized natural rubber with dodecanedioic acid and the mechanism of its acceleration by 1,2-dimethylimidazole (DMI) are investigated using nuclear magnetic resonance spectroscopy. Solid-state NMR experiments (1H, 1H → 13C cross-polarization and 13C direct polarization) are used to analyse crosslinked samples, whereas 13C solution NMR provides information about liquid epoxidized rubber species. 13C solid-state NMR demonstrates the ester nature of the crosslinks, and confirms that DMI acts through the formation of imidazolium dicarboxylate species, well dispersed in the elastomeric matrix. After synthesis of a low molecular weight epoxidized rubber and subsequent reaction with a monofunctional carboxylic acid in the presence of DMI, a solution NMR study reveals that the imidazolium dicarboxylate would react preferentially at the less substituted side of the epoxy site.