Increase in serum 25-hydroxyvitamin-D3 in humans after solar exposure under natural conditions compared to artificial UVB exposure of hands and face†
Pameli
Datta
*a,
Morten Karsten
Bogh
a,
Peter
Olsen
a,
Pia
Eriksen
a,
Anne Vibeke
Schmedes
b,
Mette Marie-Louise
Grage
c,
Peter Alshede
Philipsen
a and
Hans Christian
Wulf
a
aDepartment of Dermatology, Copenhagen University Hospital, Bispebjerg, Bispebjerg Bakke 23, 2400 Copenhagen, NV, Denmark. E-mail: Pameli@mail.dk; Tel: (+45) 35 31 62 73
bDepartment of Clinical Biochemistry, Lillebaelt Hospital, 7100 Vejle, Denmark
cDanish Meteorological Institute, Lyngbyvej 100, 2100 Copenhagen O, Denmark
First published on 5th July 2012
Abstract
Vitamin D studies are often performed under controlled laboratory conditions and the findings may be difficult to translate to natural conditions. We aimed to determine and compare the doses of natural solar ultraviolet radiation (UVR) with doses of artificial UVB radiation of hands and face needed to increase serum 25-hydroxyvitamin-D3 (25(OH)D). Furthermore, we aimed to investigate the natural course of 25(OH)D due to solar exposure from April to September. 46 Caucasian volunteers were included. 17 volunteers received solar UVR (Group 1) in their natural Danish environment. Individual daily solar UVR doses in standard erythema doses (SEDs) were determined with personal wristwatch UV-dosimeters. 29 volunteers (Group 2) received artificial UVB doses of 6 SEDs (N = 14) and 3 SEDs (N = 15) on hands and face during late-winter/early-spring when outdoor UVB is negligible. 25(OH)D-levels were determined around every second week during study periods. Solar-UVR doses and sun-exposure diaries with information of sun-exposed areas were available from 8 volunteers and used for comparison with artificial UVB doses. However no significant solar-induced Δ25(OH)D was observed when sun-exposed areas were limited to hands and face. Instead the earliest period (week 17–19) with significant Δ25(OH)D, occurring after a mean of 2 days of sun-exposing more than hands and face, was used to estimate an approximate UVR dose required to increase 25(OH)D. This estimate resulted in a dose of 4.1 solar SEDs required to increase 25(OH)D by 1 nmol l−1. The artificial dose of 6 SEDs of only hands and face significantly increased 25(OH)D and resulted in a dose of 0.52 SEDs required to increase 25(OH)D significantly by 1 nmol l−1. Artificial UVB was thus at least 8 times more efficient in increasing 25(OH)D than solar UVR at a UV-exposed area consisting of approximately hands and face. Solar UVR exposure of larger areas may lead to enhanced efficacy but was not relevant for this comparison. Significant solar-induced Δ25(OH)D was present earliest at April 8, maximal by early August and decreased by late August.
Introduction
Solar ultraviolet radiation (UVR) as well as artificial ultraviolet B (UVB) radiation (280–320 nm) increases vitamin D levels in humans.1–5 Vitamin D is crucial in maintaining bone health and in the prevention of fractures and falls, and the number of diseases that may be associated to a deficiency is increasing rapidly.6–10The main amount of vitamin D is synthesized in the skin after solar UVB exposure and only supplemented by dietary intake. The level of UVB in sunlight is low during the winter season in large parts of the world. At the same time the temperature limits the exposed body surface area mainly to the face and hands and no significant vitamin D production occurs during November–February in Denmark.11–13 This results in seasonal variation in vitamin D levels in countries at high latitudes and with long winters and is reflected in the seasonal difference observed in the vitamin D metabolites 25-hydroxyvitamin D3 (25(OH)D).14–16
We have previously demonstrated that a small suberythemal dose (1 standard erythema dose (SED)) of artificial UVB exposure to 88% body surface area every second week is sufficient to maintain summer vitamin D status in winter.17 However, in Denmark during the winter half-year as a rule only the face and hands are exposed to sunlight. It is uncertain whether solar UVR exposure of only hands and face during spring is sufficient to cause a significant increase in vitamin D. There have been several studies investigating the effects of either artificial UVB or solar UVR radiation of different body surface areas on 25(OH)D levels.2,4,17–19 To our knowledge, there have not been any studies comparing the two sources so that laboratory experiments with UVB radiation can be translated into sunlight doses outdoors.
This study sought to investigate the doses of solar and artificial UVB exposure of face and hands that would induce an increase in 25(OH)D and compare these doses in efficacy. In addition, we aimed to determine at what time of year significant 25(OH)D synthesis is induced and maximum 25(OH)D level is achieved and how this is related to UVR dose and sun-exposed body surface area.
Materials and methods
Volunteers and procedure: This study was an open controlled clinical trial conducted at Bispebjerg University Hospital, Denmark (56°N) in February to September 2008 (week 8–35, a total of 28 weeks), March to April 2011 (10 days) and February 2012 (10 days). The Danish Medical Ethics Committee approved the study protocols (H-B-2007-100, H-2-2010-097). The study was performed in accordance with the Declaration of Helsinki, and written informed consent was obtained from all volunteers. 38 healthy Caucasian volunteers residing in Denmark and of Danish descent were recruited. 36 unique volunteers completed the study, with 10 volunteers being studied in two sessions in different years, to give a total of 46 sets of results. All volunteers were skin-type I–IV according to Fitzpatrick's classification.20 17 of the volunteers participated in Group 1 and received solar UVR in their natural environment. 19 of the volunteers participated in Group 2 and received 2 different doses of artificial UVB. 25(OH)D were measured in both groups at baseline, approximately every second week throughout the study period and at study-end.Group 1
The volunteers were a homogeneous group of 17 medical students (female 13 and male 4, mean age 26.4, range 23–30). The ambient UVR was determined using personal electronic UVR dosimeters in a watch and worn on the left wrist from March to September 2008 (week of year: 13–35, 23 weeks). Of these, 10 volunteers consistently wore the UVR dosimeters. In the period from late March to late May there were 250 measurement days (43.6%) out of 574 possible days. There were 121 days with positive measurement (21.1%). Technical problems with the dosimeters were responsible for a loss of 12.1% of the data.Sun exposure diary data were available for 1072 days (mean 134 days per volunteer, range 116–153) out of a total of 1075 days. We used information retrieved from the sun exposure diaries to determine when more than hands and face were exposed. Furthermore, we used data from the Danish Meteorological Institute (DMI) with daily recordings of maximum temperatures (°Celsius), hours of sunshine and cumulative daily UVR doses (SEDs) to determine if there was a consistency between these data and the information from the sun exposure diaries.
Group 2
Group 2 consisted of 19 volunteers (female 14, male 5) with a mean age of 40 (range 19–68). This corresponded to 29 volunteer years as 10 persons participated 2 years. They received 4 sessions of artificial UVB of hands and face with either 1.5 SEDs (N = 14) or 0.75 SEDs (N = 15) per session at intervals of 2 or 3 days during early spring 2011 or late winter 2012. The study period was 10 days. The inclusion criterion was: age 18–70. The exclusion criteria were: (1) skin disease on irradiated surfaces; (2) intake of cholesterol-lowering or photosensitizing medication; (3) pregnancy; (4) drug addiction; (5) psychiatric disease; (6) solarium or sun holidays south of latitude 45°N during the study period and a month prior to study start; (7) supplementary vitamin D one month prior to baseline and during the study period.| Visit | Period | Week | N | 25(OH)D | P-value |
|---|---|---|---|---|---|
| 1 | 23 February (20–27/2) | 8.3 | 16 | 37.1 (19.4–92.8) | — |
| 2 | 12 March (10–17/3) | 10.9 | 17 | 38.7 (16.4–103.4) | 0.910 |
| 3 | 26 March (24–28/3) | 12.9 | 17 | 40.6 (20.1–111.5) | 0.196 |
| 4 | 8 April (7–10/4) | 14.7 | 17 | 43.5 (17.6–111.1) | 0.044 |
| 5 | 24 April (21–29/4) | 17.0 | 16 | 47.1 (18.8–107.2) | 0.023 |
| 6 | 9 May (6–14/5) | 19.1 | 13 | 52.7 (23.2–83.4) | 0.010 |
| 7 | 21 May (19–23/5) | 20.9 | 7 | 75.1 (22.0–122.8) | 0.028 |
| 8 | 2 June (28/5–10/6) | 22.6 | 17 | 80.6 (51.1–140.7) | <0.001 |
| 9 | 24 June (20/6–2/7) | 25.7 | 17 | 87.4 (46.7–169.0) | <0.001 |
| 10 | 4 August (4–6/8) | 31.6 | 9 | 99.7 (62.2–133.6) | 0.012 |
| 11 | 29 August (25/8–5/9) | 35.1 | 10 | 89.9 (59.0–133.5) | 0.008 |
Peripheral venous blood samples were centrifuged at 5000 rpm for at least 10 min and serum was stored at below −20 °C within 6 h of sampling. Serum samples were prepared through protein precipitation with acetonitrile and purification using solid phase extraction. The eluate was used for analysis of serum 25(OH)D concentration on a liquid chromatography-tandem mass spectrometer (LC-MS/MS) as described elsewhere.18 At least a triplet analysis of samples from each volunteer was performed to minimize variance. At 20–222 nmol l−1 the total relative standard deviation (SD) for 25(OH)D determinations varied between 4.9%–14.1%. At 50 nmol l−1 the total relative SD was maximally 14.1%.
Results
The natural course of the mean serum 25(OH)D levels in the 17 volunteers exposed to solar UVR is illustrated in Fig. 1(A). Table 1 shows the visit dates, weeks and corresponding 25(OH)D levels.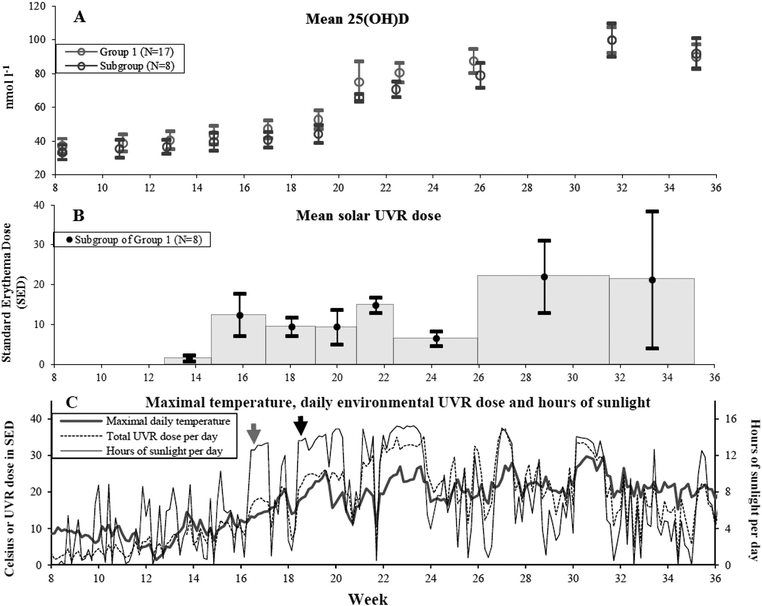 | ||
| Fig. 1 (A) Mean 25(OH)D level in nmol l−1 and 1 standard error of mean (SEM) at the 11 visits at different weeks of the year for Group 1 volunteers who received solar UVR in their natural environment from February 20 to September 5 in 2008. Grey circles: Group 1 (N = 17) 25(OH)D values. Black circles: subgroup of Group 1 (N = 8) 25(OH)D values. Only the subgroup had available individual UVR doses. (B) In the subgroup of Group 1 (n = 8) mean UVR doses in standard erythema dose (SED) in between visits are indicated by black dots and shown in the middle of the period with error bars of 1 SEM. The grey histogram indicates the period for which the dose was received. The first measurement was available from week 13 (March 26) and henceforth. (C) Data from February 20 to September 5 in 2008 retrieved from the Danish Meteorological Institute are shown here. Grey line: maximum daily temperature in °Celsius. Black line: hours of sunlight per day. Dotted line: total environmental measured UVR dose per day in SED. Around April 19 there is a clear increase in hours of sunlight per day indicated by the grey arrow but the maximal daily temperature is still below 20 °C. At this time some volunteers started to expose more than hands and face to sunlight. From May 1 to May 10 the maximal daily temperature is more frequently around 20–25 °C which is indicated by the black arrow. Hours of sunlight and UVR dose per day are also increased. In this period all volunteers in the subgroup (N = 8) of Group 1 had exposed more than hands and face to sunlight and started sunbathing. All three panels have a common x-axis showing the study period in weeks. | ||
Solar environmental UVR and artificial UVB required to induce an increase in 25(OH)D
Sun exposure diaries were available from 8 volunteers of the 10 volunteers with individual solar UVR doses shown in Fig. 1(B). As the sun exposure diaries provided important information about the size of the sun-exposed area we decided to use this subgroup of 8 volunteers to determine the solar UVR required to increase 25(OH)D. An overview of how data were analysed is summarised in the flowchart in Fig. 2.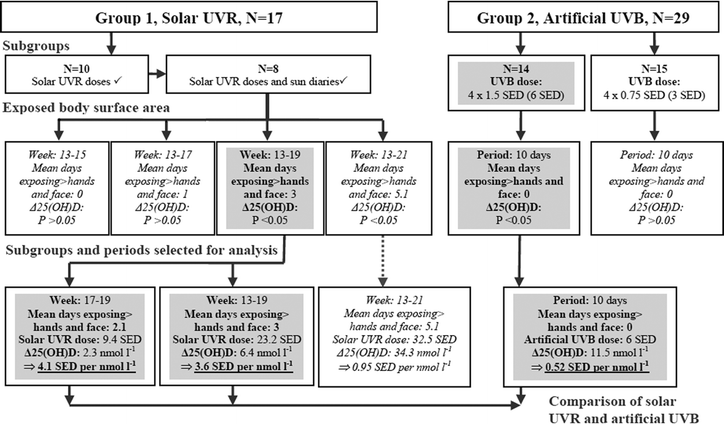 | ||
| Fig. 2 Approach to estimate required solar UVR and artificial UVB to increase 25(OH)D significantly in as comparable sizes of UV exposed areas as possible. 17 volunteers in Group 1 were exposed to solar UVR in their natural environment. Sun exposure diaries, individual solar UVR doses and 25(OH)D levels were available for a subgroup (N = 8). UVR doses were measured from March and henceforth. The sun-exposed areas are more or less restricted to hands and face during spring (week: 13–21, see Table 1 for exact visit dates and weeks) and were thus selected for analysis. Group 2 received artificial UVB on hands and face with a total dose of either 6 or 3 SEDs during late winter/early spring when outdoor UVB is negligible. In Group 1 there was no significant 25(OH)D increase when sun-exposed areas were restricted to hands and face. Comparison between the two sources of UV was performed when the earliest significant increase in 25(OH)D was present. Boxes with italic text indicate UV doses resulting in insignificant Δ25(OH)D or periods with unsuitable UV-exposed areas for comparison. Grey boxes indicate significant Δ25(OH)D and periods used for comparisons made between solar and artificial UV. Artificial UVB was at least 8 times more efficient in increasing 25(OH)D than solar UVR when the UV-exposed areas were more or less restricted to hands and face (see Table 3). | ||
Solar UVR doses were available from week 13–35 (visit 3–11). Sun exposure diaries showed that until week 15 the sun exposed area was restricted to hands and face. In this period, the increase of 25(OH)D compared to baseline was not significant using the Wilcoxon signed rank test as shown in Table 2. Linear regression analysis of these 8 volunteers in this period did not show a significant daily increase in 25(OH)D from (P = 0.383) after a UVR dose of 1.5 SEDs either. As there was no significant increase in 25(OH)D in periods when only hands and face were sun-exposed, the exact solar UVR dose required to increase 25(OH)D by 1 nmol l−1 under the predefined exposed area size could not be precisely determined.
| Visit | Week of year | ΔD | P-value | UVR dose | Mean days of exposing > hands and face |
|---|---|---|---|---|---|
| 1 | 8,3 | — | — | — | — |
| 2 | 10,7 | −2.2 | 0.401 | — | — |
| 3 | 12,7 | 1.2 | 0.484 | — | — |
| 4 | 14,7 | 3.0 | 0.093 | 1.5 (0.1–6.1) | 0 (0) |
| 5 | 17,0 | 1.1 | 0.327 | 12.3 (1.8–30.2) | 1 (8) |
| 6 | 19,1 | 2.3 | 0.043 | 9.4 (4.7–17.8) | 3 (24) |
| 7 | 20,9 | 27.9 | 0.109 | 9.3 (4.9–13.6) | 5.1 (41) |
| 8 | 22,4 | 17.7 | 0.012 | 14.8 (10.0–24.4) | 5.6 (45) |
| 9 | 26,0 | 8.4 | 0.012 | 6.4 (0.8–12.7) | 10.3 (82) |
| 10 | 31,6 | 21.0 | 0.018 | 21.9 (6.8–64.5) | 18.5 (148) |
| 11 | 35,1 | −5.5 | 0.012 | 21.2 (0.2–124.1) | 19.5 (156) |
Instead the required solar UVR dose to increase 25(OH)D by 1 nmol l−1 on an exposed area of approximately hands and face, including the least number of days where more than hands and face were sun-exposed as possible, was estimated. Hence the earliest period with significant 25(OH)D level was selected. Significant Δ25(OH)D was observed earliest in week 19. The period between week 17–19 included a mean of 2 days of exposing more than hands and face to sunlight making the exposed area fairly comparable with Group 2. Furthermore the length of the period (2 weeks) was also similar to the period in which Group 2 received the artificial UVB dose (10 days). In this time interval (week 17–19) they received a mean of 9.4 SEDs and increased 25(OH)D by a mean of 2.3 nmol l−1 resulting in a UVR dose of 4.1 SEDs required to increase by 1 nmol l−1 as shown in Table 3 and Fig. 2.
| Group | Visit | Week | N | UV-dose | 25(OH)D at baseline | 25(OH)D after irradiation | ΔD | P-value | Mean days of exposing > hands and face | UV dose to increase 1 nmol l−1 25(OH)D | Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solar UVR | |||||||||||
| 1 | 5–6 | 17–19 | 8 | 9.4 | — | — | 2.3 | 0.043* | 2.4 | 4.1 | ∼8 |
| 1 | 3–6 | 13–19 | 8 | 23.2 | — | — | 23.2 | 0.043* | 3.0 | 3.6 | ∼7 |
| 1 | 3–7 | 13–21 | 8 | 32.5 | — | — | 34.3 | 0.109* | 5.1 | 1.0 | ∼2 |
| Artificial UVB | |||||||||||
| 2 | — | 14 | 6 | 54.6 (28.2–103.3) | 66.1 (35.8–126.2) | 11.5 | 0.002 | 0 | 0.52 | 1 | |
| 2 | — | 15 | 3 | 58.3 (31.3–86.1) | 61.7 (39.2–97.0) | 3.40 | 0.394 | 0 | — | — | |
Additionally we have supplemented with estimates from week 13–19 (6 weeks) which provides a longer observation period. This period included an increased mean of 3 days of exposing more than hands and face to sunlight and resulted in a solar dose of 3.6 SEDs required to increase 25(OH)D by 1 nmol l−1.
When the observation period was extended further to week 13–21 (8 weeks) the mean days of exposing more than hands and face was increased from 2 to 5.1 and the solar UVR dose required to increase 25(OH)D by 1 nmol l−1 was reduced to 1.0 (Table 3). However in this period the sun-exposed area was no longer comparable to the areas exposed to artificial UVB and not relevant to this study. But it shows that the efficacy of inducing 25(OH)D increases when the sun-exposed surface area is enlarged.
The sun exposure diaries showed that all volunteers began to expose more than hands and face regularly from May 1 to May 10 just prior to week 19. This coincides with the first present significant increase in 25(OH)D compared to baseline using the Wilcoxon signed rank test as shown in Table 2. At the same time there is a clear rise in the daily maximum temperature and hours of sun, as shown in Fig. 1(C).
Group 2 had a significant increase in mean 25(OH)D by 11.5 nmol l−1 (P = 0.002) after a UVB dose of 6 SEDs of hands and face only as shown in Table 3. Hence, the required SEDs to increase 25(OH)D by 1 nmol l−1 were 0.52 SEDs at this dose. This means that artificial UVB in this study was estimated to be at least 8 times more effective in increasing 25(OH)D than solar UVR under conditions favouring the efficacy of the solar UVR.
The volunteers receiving 3 SEDs lead to an insignificant tendency of increase of 3.4 nmol l−1 (P = 0.113). There was a significant difference between the Δ25(OH)D after 6 SEDs and 3 SEDs (P = 0.046).
At what time of year does a significant increase of 25(OH)D due to solar UVR occur?
Mean baseline 25(OH)D levels were 37.1 nmol l−1 in Group 1 and 33.4 nmol l−1 in the subgroup of 8. These baseline levels were increased significantly for Group 1 by April 8 (P = 0.044) and for the subgroup of 8 by May 9 after a total UVR dose of 23.2 SEDs (P = 0.043). There was good accordance between what was reported in the sun exposure diaries and the climate data shown in Fig. 1(C) indicating relatively good reliability regarding assessment of sun-exposed body surface area. These data combined indicated that significant increases in 25(OH)D occur only when areas larger than hands and face are sun-exposed frequently and are concurrent with a clear rise in daily maximal temperature and hours of sunlight (Fig. 1(C)).A maximum level of 99.7 nmol l−1 25(OH)D due to solar UVR in Group 1 was achieved at August 4. Between August 4 and September 5 the 8 volunteers received an average of 21.2 SEDs but 25(OH)D still declined. In this period (week 32–35) sun exposure diaries show a drop in the mean days of sunbathing from 5.4 days (week 26–32) to 1 (week 32–35). Similarly sun exposure of more than hands and face drops from a mean of 8.3 days (week 26–32) to 2.1 (week 32–35).
Discussion
Solar environmental UVR and artificial UVB required to induce an increase in 25(OH)D
The main purpose of this study was to compare the efficacy of inducing vitamin D synthesis between solar UVR and artificial UVB. This was done to provide a means of translating artificial UVB doses into real life solar UVR doses. One of the challenges to overcome is to keep the UV exposed body surface areas comparable. The size of solar exposed body surface area is highly variable with time of the day, season and individual clothing habits. In the winter half-year at high latitudes when temperatures are low the sun-exposed areas are usually limited to hands and face and subsequently more reliably defined and therefore suited for this study. Under these conditions it was our objective to determine the UVR dose needed to increase 25(OH)D by 1 nmol l−1 and compare it to an equivalent artificial required UVB dose.However in this study solar UVR exposure of hands and face in Group 1 did not increase 25(OH)D significantly. Significant 25(OH)D was observed after an artificial dose of 6 SEDs on an area restricted to hands and face in Group 2. Consequently an exact relationship between the efficacy of solar UVR and artificial UVB based on these predefined exposure areas could not be established.
Significant increase in 25(OH)D in Group 1 occurred earliest in week 19 after a mean of 25.2 solar SEDs and a mean of 3 days of exposing much more than hands and face to sunlight. The period between week 17–19 was used for comparison instead because the days of exposing more than hands and face were kept to a minimum and the length of the period was more comparable to the conditions of Group 2 receiving artificial UVB. But as a result of this, the area exposed in Group 1 receiving solar UVR to some extent exceeded the area exposed in Group 2 receiving artificial UVB. The size of exposed body surface area has previously been shown to influence Δ25(OH)D at different UVB doses when the exposed area is relatively small as in this study.4 Consequently this approach introduces a bias that overestimates the efficacy of solar UVR substantially compared to artificial UVB. Based on these results we can conclude that solar UVR exposure is at least 8 times less effective in inducing 25(OH)D synthesis compared to artificial UVB. When the UV exposed area was enlarged in the solar group the difference in efficacy was reduced. However the purpose of this study was to make a comparison under as equal conditions as possible. Whether the difference in efficacy of increasing 25(OH)D is sustained at larger UV doses and larger exposed areas remains to be resolved.
Artificial UVB can be irradiated on all body surface areas with an equal intensity whereas irradiation from sunlight depends on season, body position and on what time of the day you are exposed to sunlight (sun altitude). Solar UVR is also considered to be a little less effective as UVA contributes to SED but not to 25(OH)D synthesis. The difference in efficacy of inducing vitamin D synthesis found in this study is therefore not in contrast to the general notion.
This part of the study was limited by that only a subgroup of 8 volunteers in Group 1 had both sun exposure diaries and UVR doses available and used for the comparison. Among the volunteers with UVR doses there were only 43.6% measurement days available and of these 21.1% days with a positive measurement. This could either mean that compliance was not optimal and that many UVR exposure days have not been recorded. In this case, the measured solar SED doses are underestimates and this would emphasize our conclusion that artificial UVB is far more effective in inducing 25(OH)D than environmental UVR. Or this could be explained by the fact that the medical students were studying for exams resulting in many days with no UVR doses. The fact that the sun exposure diaries do not report the sun-exposed area precisely contributes to some uncertainty to the exact time when the predefined sun-exposed area was expanded. The diaries only give information in regards to when the exposed area was increased considerably, i.e. when the shoulders or upper body were exposed. For this reason we underpinned our sun exposure diaries information by using national climate data consisting of daily maximum temperatures, hours of sunshine and accumulated UVR doses. There was a good consensus between the information from these two sources, indicating that our estimate of sun-exposed areas was fairly acceptable although not precise. It is important to bear in mind in this context that cultural differences in sun exposure habits exist. What is considered sun bathing weather and temperature in one country does not necessarily apply to another. Rather than using general environmental based UVR doses we used personal UVR doses in this study. These measurements take individual differences in sun exposure habits into account. However personal UV dosimeters worn on the wrist record more reliable doses of UVR received on the hands than on the face. Thieden and colleagues have previously shown that wrist, shoulder, upper arm and chest receive about 50%, 64%, 50% and 34% respectively of the UVR dose received by the top of the head.28 The dose received on the face may be better represented as the mean value of the dose received on the upper arm and the shoulder corresponding to 57% of the dose received by the top of the head. Nonetheless, this can only account for a minimal underestimation of the solar SED dose.
At what time of year does a significant increase of 25(OH)D due to solar UVR occur?
A previous simulation study found that in theory it is possible to acquire a UVR dose adequate for vitamin D synthesis in spring for all skin types under various atmospheric and surface conditions at the maximal latitude of 54°N when hands, face and arms (25% of body surface area) are sun-exposed. This would be equivalent to between 26–152 min of sun exposure depending on skin type (I–IV) at March 21 and 8–13 min at June 21.29 Our study was located 2° further north, and confirms under natural conditions the difficulties in achieving adequate UVR doses on small body surface areas to increase vitamin D during spring as described by the simulation study. In other words, it may in theory be possible for 25(OH)D synthesis to start as soon as March but in real life not likely to happen with the recommended sun-exposure times when daily temperature is low and sun-exposed areas are restricted to hands and face. The earliest point at which positive significant Δ25(OH)D takes place is April 8 in our study. However we have no detailed knowledge of their sun behaviour that possibly could include using solarium or holidays with sunbathing. But in the subgroup of 8 volunteers with detailed sun-exposure diaries positive significant 25(OH)D synthesis occurred as late as May 9 without the use of solarium or sun holidays abroad prior to this. Apart from differences in individual sun habits the onset of significant 25(OH)D may also depend on variations in maximum daily temperatures and daily hours of sun from year to year.In a Norwegian study performed at 68.2°N, 15 volunteers were exposed to a minimum of 20 min of sunlight only to the face from February to mid-April. Environmental measurements of UVR from a spectrophotometer located close to their work were used. There was no significant increase in 25(OH)D.19 It clearly supports our perhaps most surprising result that no significant increase in Δ25(OH)D is induced as long as the sun-exposed area is limited to hands and face despite relatively high doses of solar UVR (mean of 13.8 SEDs) during late spring. It is sustained even when larger areas are exposed to sun occasionally (mean of 1 day during a period of approximately 2 weeks) as observed in the Group 1 subgroup of 8 volunteers between week 15–17.
A maximum level of 25(OH)D due to solar UVR was achieved in August 4 for Group 1 and the subgroups just after normal summer holidays. A decrease in 25(OH)D was observed in August 29 though receiving the same UVR dose (21.2 SEDs) as the previous time period. Sun exposure diaries indicate that the mean days of sun-exposing more than hands and face and sunbathing is reduced again and may in part be responsible for this.
Conclusion
An artificial UVB exposure of 6 SEDs of hands and face in Group 2 (N = 14) induces significant 25(OH)D synthesis whereas an equivalent dose of solar UVR of hands and face in a sub-group of Group 1 (N = 8) does not. Consequently a direct comparison was not possible. Instead the required solar UVR dose to increase 25(OH)D by 1 nmol l−1 on an exposed area consisting of approximately hands and face was estimated.Artificial UVB was thus found to be at least 8 times more effective in inducing 25(OH)D synthesis than solar UVR using approximately the same size of UV-exposed areas. This estimate favours the efficacy of solar UVR due to the presence of sun-exposed areas exceeding hands and face occasionally. Significant increase in 25(OH)D level required a mean of 25.3 solar SEDs and a mean of 3 days of exposing much more than hands and face and took place in week 19. The efficacy of solar UVR increased markedly when the size of the sun-exposed areas was enlarged more regularly (week 13–21) but were then not comparable with the artificial dose.
The earliest onset of significant 25(OH)D increase was at April 8 (week 15) in this study. The maximum level of 25(OH)D was 99.7 nmol l−1 in August 4 (week 32). Subsequently (week 35) the mean 25(OH)D level decreased probably due to a decrease in the size of the exposed body surface area.
Acknowledgements
This work has only been supported by Copenhagen University Hospital, Bispebjerg. We thank the volunteers for taking part in this study, Jacob Heydenreich for his technical assistance in this project and Copenhagen University Hospital, Bispebjerg for providing location and equipment.References
- J. A. MacLaughlin, R. R. Anderson and M. F. Holick, Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin, Science, 1982, 216, 1001–1003 CAS
.
- M. K. Bogh, A. V. Schmedes, P. A. Philipsen, E. Thieden and H. C. Wulf, Vitamin D production depends on ultraviolet-B dose but not on dose rate: a randomized controlled trial, Exp. Dermatol., 2011, 20, 14–18 CrossRef CAS
.
- L. A. Armas, S. Dowell, M. Akhter, S. Duthuluru, C. Huerter, B. W. Hollis, R. Lund and R. P. Heaney, Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color, J. Am. Acad. Dermatol., 2007, 57, 588–593 CrossRef
.
- M. K. Bogh, A. V. Schmedes, P. A. Philipsen, E. Thieden and H. C. Wulf, Interdependence between body surface area and ultraviolet B dose in vitamin D production: a randomized controlled trial, Br. J. Dermatol., 2011, 164, 163–169 CrossRef CAS
.
- Commision Internationale de l'Eclairage (CIE), Action spectrum for the production of previtamin D3 in human skin, Technical Report CIE, 2006, 174, 1–12.
- H. A. Bischoff-Ferrari, D. P. Kiel, B. Dawson-Hughes, J. E. Orav, R. Li, D. Spiegelman, T. Dietrich and W. C. Willett, Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults, J. Bone Miner. Res., 2009, 24, 935–942 CrossRef CAS
.
- H. A. Bischoff-Ferrari, B. Dawson-Hughes, H. B. Staehelin, J. E. Orav, A. E. Stuck, R. Theiler, J. B. Wong, A. Egli, D. P. Kiel and J. Henschkowski, Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials, Br. Med. J., 2009, 339, 1–11 CrossRef
.
- H. A. Bischoff-Ferrari, W. C. Willett, J. B. Wong, A. E. Stuck, H. B. Staehelin, E. J. Orav, A. Thoma, D. P. Kiel and J. Henschkowski, Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials, Arch. Intern. Med., 2009, 169, 551–561 CrossRef CAS
.
- C. L. Wagner and F. R. Greer, Prevention of rickets and vitamin D deficiency in infants, children, and adolescents, Pediatrics, 2008, 122, 1142–1152 CrossRef
.
- A. Cranney, T. Horsley, S. O'Donnell, H. Weiler, L. Puil, D. Ooi, S. Atkinson, L. Ward, D. Moher, D. Hanley, M. Fang, F. Yazdi, C. Garritty, M. Sampson, N. Barrowman, A. Tsertsvadze and V. Mamaladze, Effectiveness and safety of vitamin D in relation to bone health, Evid. Rep. Technol. Assess. (Full. Rep.), 2007, 1–235 Search PubMed
.
- M. F. Holick, McCollum Award Lecture, 1994: vitamin D – new horizons for the 21st century, Am. J. Clin. Nutr., 1994, 60, 619–630 CAS
.
- E. Thieden, P. A. Philipsen, J. Heydenreich and H. C. Wulf, Vitamin D level in summer and winter related to measured UVR exposure and behavior, Photochem. Photobiol., 2009, 85, 1480–1484 CrossRef CAS
.
- A. R. Webb, B. R. DeCosta and M. F. Holick, Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation, J. Clin. Endocrinol. Metab., 1989, 68, 882–887 CrossRef CAS
.
- P. S. Davies, C. J. Bates, T. J. Cole, A. Prentice and P. C. Clarke, Vitamin D: seasonal and regional differences in preschool children in Great Britain, Eur. J. Clin. Nutr., 1999, 53, 195–198 CAS
.
- S. S. Harris and B. Dawson-Hughes, Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women, Am. J. Clin. Nutr., 1998, 67, 1232–1236 CAS
.
- J. R. Juttmann, T. J. Visser, C. Buurman, K. E. de and J. C. Birkenhager, Seasonal fluctuations in serum concentrations of vitamin D metabolites in normal subjects, Br. Med. J., 1981, 282, 1349–1352 CrossRef CAS
.
- M. K. Bogh, A. V. Schmedes, P. A. Philipsen, E. Thieden and H. C. Wulf, A small suberythemal UVB dose every second week is sufficient to maintain summer vitamin D levels: a randomized controlled trial, Br. J. Dermatol., 2011, 166, 430–433 CrossRef
.
- M. K. Bogh, A. V. Schmedes, P. A. Philipsen, E. Thieden and H. C. Wulf, Vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation, J. Invest. Dermatol., 2010, 26, 546–553 CrossRef
.
- K. Edvardsen, M. Brustad, O. Engelsen and L. Aksnes, The solar UV radiation level needed for cutaneous production of vitamin D3 in the face. A study conducted among subjects living at a high latitude (68 degrees N), Photochem. Photobiol. Sci., 2007, 6, 57–62 CAS
.
- T. B. Fitzpatrick, The validity and practicality of sun-reactive skin types I through VI, Arch. Dermatol., 1988, 124, 869–871 CAS
.
- A. McKinlay and B. Diffey, A reference action spectrum for ultraviolet induced erythema in human skin, 1987, 6, 17–22.
- J. Heydenreich and H. C. Wulf, Miniature personal electronic UVR dosimeter with erythema response and time-stamped readings in a wristwatch, Photochem. Photobiol., 2005, 81, 1138–1144 CrossRef CAS
.
- H. C. Wulf, J. Heydenreich and P. A. Philipsen, Variables in full-body ultraviolet B treatment of skin diseases, Photodermatol., Photoimmunol. Photomed., 2010, 26, 165–169 CrossRef
.
- B. L. Diffey, C. T. Jansen, F. Urbach and H. C. Wulf, The standard erythema dose: a new photobiological concept, Photodermatol., Photoimmunol. Photomed., 1997, 13, 64–66 CrossRef CAS
.
- Commision Internationale de l'Eclairage (CIE), Standard erythema dose, 1997, 125, 1–5.
- H. C. Wulf and J. Lock-Andersen, Short report: standard erythema dose, Skin Res. Technol., 1996, 4, 192–192 Search PubMed
.
- J. Lock-Andersen and H. C. Wulf, Threshold level for measurement of UV sensitivity: reproducibility of phototest, Photodermatol., Photoimmunol. Photomed., 1996, 12, 154–161 CrossRef CAS
.
- E. Thieden, M. S. Agren and H. C. Wulf, The wrist is a reliable body site for personal dosimetry of ultraviolet radiation, Photodermatol., Photoimmunol. Photomed., 2000, 16, 57–61 CAS
.
- A. R. Webb and O. Engelsen, Calculated ultraviolet exposure levels for a healthy vitamin D status, Photochem. Photobiol., 2006, 82, 1697–1703 CAS
.
Footnote |
| † Contribution to the Vitamin D Update collected papers. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2012 |
