UVA filters in sun-protection products: regulatory and biological aspects†‡
A.
Fourtanier
a,
D.
Moyal
a and
S.
Seite
b
aL'Oréal Research & Innovation, Asnières, France
bLa Roche-Posay Pharmaceutical Laboratories, 110 Avenue Henri Barbusse, 92602, Asnières Cedex, France. E-mail: sseite@dca.loreal.com; Fax: (+33) 1 46 88 29 22; Tel: (+33) 1 46 88 65 44
First published on 8th September 2011
Abstract
This review of published in vitro and in vivo studies concerning the biological effects of ultraviolet A (UVA; 320–400 nm) radiation illustrates the evidence for combining UVA and UVB filters in sun-protection products. These data have led to the development of new sunscreens as well as methods to evaluate their efficacy. After listing the UVA filters available and briefly noting the requirements for a high SPF, broad-spectrum sunscreen, the methods for evaluating the level of UVA protection will be described. This article also summarizes several studies looking at the prevention of erythema, pigmentation, DNA damage, photoimmunosuppression, photoaging and photodermatoses. These data demonstrate in vitro and in vivo that only well-balanced UVA-UVB sunscreens, absorbing over the entire UV spectrum are able to prevent or significantly reduce the associated biological damage.
Introduction
The solar UV radiation which reaches the earth's surface is a combination of UVB (290–320 nm) and UVA (320–400 nm) radiation. UV irradiation depends on many parameters including latitude, the season, the time of day, the meteorological conditions, and the ozone layer. On a summer's day, the UV energy received (daily dose) is comprised of approximately 3.5% UVB and 96.5% UVA. For example, on a mid summers' day, in the south of France the maximum ambient daily UV erythemal dose, is around 22 MEDs and the maximum UVA dose is around 137 J cm−2.1Short and long-term adverse effects to the skin are induced by both acute and repeated sun-exposure. Such effects include sunburn, pigmentation, immunosuppression, photoaging, photodermatoses, and skin cancers.
For the past several decades, UVB radiation (290–320 nm) of the solar spectrum was considered to be deleterious, while UVA radiation (320–400 nn) was generally believed to be innocuous. However, with the availability of high intensity artificial UVA sources, it has been demonstrated that UVA radiation penetrates deeper into the skin2 causing a wide variety of damaging biological effects. UVA radiation produces mainly reactive oxygen species (ROS) through the interaction with endogenous and exogenous chromophores. These ROS cause damage to DNA, cells, vessels and tissues.3–7 Like UVB, UVA has been implicated in immune system depression and in the development of skin cancer, including melanoma.8,9 Photoallergies and phototoxic reactions as well as photodermatoses are mainly UVA induced.10–12 Furthermore, we recently published a review paper describing biological damage induced by cumulative sub-erythemal UVA exposure.13 So it is important to reduce UVA exposure to the skin by using sunscreens containing an association of UVA and UVB filters.
In response to the growing awareness of the additional damage caused by UVA radiation, various UVA filters are now available for formulation in Europe and USA. The different types of filters have specific requirements in order to correctly formulate effective sun protection products. Methods to measure the level of the UVA protection obtained have also evolved. To demonstrate the advantage of UVA protection, we also report on selected human in vivo or in vitro studies describing the efficacy of sunscreens with differing levels of UVA protection.
Regulatory status of UVA filters in Europe and USA
Advanced technology in the last decade, has permitted the development of many new UVA filters. However, their use has been slowed down by regulatory barriers, particularly in the USA compared with Europe or other parts of the world. The availability of effective sunscreen products on the market depends on the regulatory status of UV filters as well as on the ability to inform the consumer about the protective efficacy of these products with appropriate labelling indicating both sun protection factor (SPF) and UVA protection level (UVA-PF).Comparing the number of UVA filters or broad-spectrum UVB/UVA filters developed and authorised in Europe (Cosmetics Directive,14 Annex VII) (Table 1) and the USA (Table 2), it is obvious that the number of authorised filters included in the monograph15 in the USA (Table 2) is quite limited. In addition, there are some limitations according to the sunscreen monograph in the use of butyl methoxy dibenzoyl methane (BMDM) also called avobenzone. The concentration is limited to 3% and combinations with some other UV filters such as titanium dioxide (TiO2), or enzulizole are not permitted.
| INCI a name (other name or abbreviation) | Maximum EU-approved concentration (%) | Maximum wavelength absorbed (nm) |
|---|---|---|
| a INCI: International Nomenclature of Cosmetic Ingredients. | ||
| Butyl methoxy dibenzoyl methane (BMDM) | 5 | 355 |
| Oxybenzone (benzophenone-3) | 6 | 288, 329 |
| Sulibenzone (benzophenone-4) | 5 | 286, 324 |
| Terephthalylidene dicamphor sulfonic acid (TDSA) | 10 | 345 |
| Drometrizole trisiloxane (DTS) | 15 | 303, 344 |
| Disodium phenyl dibenzimidazole tetrasulfonate (DPDT) | 10 | 335 |
| Diethylamino hydroxybenzoyl hexyl benzoate (DHHB) | 10 | 354 |
| Methylene bis-benzotriazolyl tetramethylbutylphenol (MBBT) | 10 | 305,360 |
| Bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT) | 10 | 310, 343 |
| Titanium dioxide (TiO2) | 25 | 295 |
| INCI a name (other name or abbreviation) | Maximum FDA-approved concentration (%) | Maximum wavelength absorbed (nm) |
|---|---|---|
| a INCI: International Nomenclature of Cosmetic Ingredients. | ||
| Avobenzone (butylmethoxydibenzoylmethane) | 3 | 357 |
| Oxybenzone (benzophenone -3) | 6 | 288, 329 |
| Sulisobenzone (benzophenone-4) | 10 | 286, 324 |
| Dioxybenzone (benzophenone-8) | 3 | 325 |
| Meradimate (menthyl anthranilate) | 5 | 336 |
| Titanium dioxide (TiO2) | 25 | 295 |
| Zinc oxide (ZnO) | 25 | 390 |
In the USA, there are two distinct regulatory processes for obtaining marketing approval for over-the-counter (OTC) products: New Drug Application (NDA) and a Time and Extent Application (TEA). A NDA is necessary to obtain marketing approval for a formula containing a new UV filter. It is also required for a new concentration of either a single or combination of previously accepted active ingredients (e.g.avobenzone 5% instead of 3%). For example, the UVA filter, terephthalylidene dicamphor sulfonic acid (TDSA) also called Mexoryl®SX has a NDA approval for each of the four formulations available. A Time and Extent Application (TEA) is a new procedure for an active ingredient where another country has already accorded marketing approval. In this procedure, the FDA accepts commercial data obtained within external markets in place of use of an authorised drug on the US market. However toxicological data requirements for a TEA remain very similar to those for NDA.
Four UVA filters with good UVA absorbance are currently eligible for evaluation through a TEA procedure (not yet finalised):
• Methylene bis-benzotriazolyl tetramethylbutylphenol (MBBT) also called Bisoctrizole (Tinosorb® M)
• Bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT) (Tinosorb® S)
• Terephthalylidene dicamphor sulfonic acid (TDSA) (Mexoryl®SX) 10%
• Drometrizole Trisiloxane (DTS) (Mexoryl®XL) 15%
Sunscreen formulation
An appropriate sunscreen product must fulfil the following critical requirements:• Provide efficient protection against UVB and UVA radiation
• Be stable to heat and to UV radiation (UVR)
• Be user-friendly to encourage frequent application and provide reliable protection
• Be cost-effective
To protect against both UVB and UVA radiation, a sunscreen product must contain a combination of active ingredients within a complex vehicle matrix. UV filters can be either organic or inorganic and act by absorption, reflection or diffusion of UVR depending on their chemical nature and physical properties.
Organic UV filters
Organic filters are active ingredients that absorb UVR energy, to a varying extent, within a specific wavelength range, according to their chemical structure. The molecular structure responsible for absorbing UV energy is called a chromophore. A chromophore consists of electrons with multiple bond sequences between atoms, generally conjugated double bonds. An absorbed UV photon contains enough energy to cause electron transfer to a higher energy orbit in the molecule. The filter that was in a low-energy state (ground state) transforms to a higher excited energy state. From an excited state, different processes can occur:• The filter molecule can be simply deactivated from its excited state and return its ground state, while releasing the absorbed energy as unnoticeable heat or fluorescence.
• Structural transformation or degradation may occur and the filter losses its absorption capacity. The filter is then said to be photo-unstable
The control of filter behaviour under UV exposure is a critical point that needs to be investigated when new sunscreen products are developed.
Methylene bis-benzotriazolyl tetramethylbutylphenol (MBBT) is a UVA filter made of organic particles that not only absorb but also scatter and reflect UV radiation.
Inorganic UV filters
Pigment grade powders of metal oxides such as titanium dioxide (TiO2) or zinc oxide (ZnO) have been used for many years in combination with organic filters to enhance protection level in the longer UVA range. Unlike organic filters, they work principally by reflecting and diffusing UVR. However, the large particle size of these powders also reflects light from the visible range of the solar spectrum leaving a white appearance on the skin. To overcome this drawback, which affects cosmetic acceptance, nanosized powders (<100 nm) of both TiO2 and ZnO were made available. Minimising the particle size changes the protective properties of titanium dioxide: the smaller particles clearly shift the protection range from the longer UVA toward the UVB. The absorption properties of these nanoparticle grades increase compared with the large particles. Zinc oxide has better absorption in the long UVA than TiO2 but is not as efficacious as organic UVA filters.When nanosized TiO2 is combined with organic UV filters, it allows high sun protection factor (SPF) products to be formulated with a lower dependence on organic UV filter concentration. In combination with organic UV filters nanosized TiO2 has a more synergistic rather than an additive effect.
Steps toward more efficacious sun protection filters
As far as UVB protection is concerned, an impressive choice of filters has been available for a number of years. The choice of available UVA filters varies from country to country and is limited in the USA as previously discussed. Inorganic pigments offer poor protection against UVA when used alone. Benzophenones are photostable but they are essentially UVB filters with some absorption in the short UVA range (peak at 328 nm).Butyl methoxy dibenzoyl methane (BMDM) has a high potency in the UVA-1 range, peaking at 358 nm. However, it undergoes significant degradation under UV exposure leading to a decrease in UVA protective efficacy. Research on the photochemistry of filters has led both to the identification of some potent photostabilisers (e.g.octocrylene or OC, BMDM) and to the development of new UVA filters that have photostable structures. Recently, in 2005, diethylamino hydroxybenzoyl hexyl benzoate (DHHB also called Uvinul® A Plus) was approved in Europe. This UVA-1 filter has UV-spectral properties similar to BMDM as well as being photostable.
In order to provide full protection in the entire UVA range, effective absorption in the short UVA range is also needed.
TDSA with a peak at 345 nm at the boundary between short and long UVA wavelengths was first approved in Europe in 1993 and then a broad UVB/UVA filter, DTS, with two peaks (303 and 344 nm), in 1998. Since 2000, four other short UVA filters, disodium phenyl dibenzimidazole tetrasulfonate (DPDT), with a peak at 334 nm, and broadband UVB/UVA MBBT and BEMT, were approved in Europe. These filters are all photostable.
Some UV filters are hydrophilic and others lipophilic; when combined, a synergistic effect can be observed. This property is used to obtain higher protection against UVB and UVA radiation.
Efficacy evaluation of sunscreen products
Sunscreen efficacy is measured firstly by its sun protection factor (SPF), which is a globally accepted index of protection from erythema following a single exposure to solar simulated radiation (SSR). SPF is determined under conditions described by authorities such as the European Commission (EC)16 or the US Food and Drug Administration (FDA),15 which has been recently updated.17Determination of UVA protection level
It is important to take into account the photoinstability of products in efficacy evaluation methods, in order to avoid overestimating the protection level.In vivo SPF test methods and in vivoUVA protection factor (UVA-PF) test methods18 take into account photodegradation due to the UV doses used to induce sunburn on human skin to determine the SPF rating and the pigmentation for the UVA-PF determination.
The EC issued a recommendation16 on September 22, 2006 to use the persistent pigment darkening (PPD) method similar to the JCIA method.18
Finally, the method is currently undergoing International Standardisation Organisation (ISO) accreditation. The publication of the ISO standard is expected towards the end of 2011.
The EC requested in its recommendation16 that all sunscreen products protect both against UVB and UVA radiation with a ratio of protection levels SPF/UVA-PF less or equal to 3 and a critical wavelength at least equal to 370 nm to ensure breadth of the UV protection in addition to the balance of UVA/UVB protection.
The European Cosmetic Industry Association (COLIPA) published in 2007 an in vitro UVA testing method19 which was validated against the in vivoPPD method with equivalent results. In addition, the critical wavelength value can be calculated using the same measurement conditions.20 The ISO is also working on this in vitro UVA standard to be published soon.
The critical wavelength determination has been proposed by the FDA recently,17 however using different technical conditions to those used by COLIPA and ISO. A critical wavelength at least equal to 370 nm was chosen by the FDA as the single criteria to claim broad-spectrum UVA protection. However, alone, for the same SPF level, this endpoint does not ensure a balance of UVA/UVB protection of at least 1/3 as defined in Europe. Products with the same SPF classified as broad-spectrum can have different levels of UVA protection.
Erythema protection
UVA radiation has a part role in the erythematous reaction induced by the sun. Therefore, to correctly protect against sunburn, sunscreens should contain UVA filters. This was demonstrated in a recent paper21 in which the protection against erythema induced by repeated sub-erythemal exposure by a UVB sunscreen and a broad-spectrum sunscreen containing UVA and UVB filters was compared. The exposures were given with a non-extreme SSR source. This energy source was developed to more accurately represent UVR that a human living in temperate latitudes could receive during daily exposure.22 The UV spectrum delivered was called UV daylight (UV-DL). The two sunscreens had a SPF of 6. The UVB product contained 4% octyl methoxycinnamate (OMC). The broad-spectrum sunscreen contained 1.65% MBBT and 0.88% BEMT. The in vitro UVA-PFs determined according to the method suggested by COLIPA in 200719 were 1.1 and 6.5, respectively, allowing a ratio SPF/UVA-PF of 5.5 and 1 respectively. They were applied on the volunteers' buttock, a non-exposed skin area. The two treated sites were exposed to 2 individually pre-determined minimal erythema doses (MED), once a day for 13 consecutive days. An untreated control site, next to the 2 others, received once a day six times smaller UV-DL dose (0.33 individual MED).Erythema was assessed using a reflectance-meter at day 6, 8 and 13. At all time points the authors showed that there was more erythema on the UVB sunscreen treated sites than in the broad-spectrum sunscreen treated sites This difference was significant, showing that even if the two products had the same SPF, the sunscreen containing a combination of UVB and UVA filters and a ratio SPR/UVA-PF of 1 gave increased protection against erythema.
More recently, we showed with a combination of UVB/UVA broad-absorbers (TDSA, BEMT) a synergistic effect to prevent erythema: a formulation containing 8% TDSA gave a SPF of 5.1 and a UVA -PF of 6, the same formulation with 8% BEMT gave a SPF of 10.8 and a UVA-PF of 6.2 and the association of 5.5% TDSA with 2.5% BEMT (total filter concentration = 8%) offered a SPF of 22.2 and a UVA-PF of 13.4. This demonstrated that it is possible to obtain a synergistic effect by combining filters; in other terms, providing a higher efficacy with the same concentration of filters.
Prevention of pigmentation
UVA-induced changes in skin colour are the result of an immediate darkening due to photooxidation of pre-existing melanin followed by a residual colour called persistent pigment darkening (PPD). This phenomenon is distinct from delayed tanning response.UVB-induced tanning is due to newly synthesized melanin. This is also observed after UVA exposure but requires a high erythemal dose or repeated sub-erythemal doses.
Even though tanned skin is desirable in many cultures of European descent, there are numerous populations, particularly in Asia and South America, for which tanned skin is undesirable. Interestingly, these ethnic groups are prone to pigmentation disorders such as lentigos and melasma.
Recently, Miyamuna et al.23 showed that UVA-induced tanning does not protect against DNA damage induced by a subsequent erythemal dose of UVR exposure. So UVA tanning may bring no benefit.
Some studies have shown that by broadening the absorption spectrum from UVB towards UVA the sunscreen efficacy is increased against sun-induced pigmentation. This was demonstrated in Asian skin24 where the ability of sunscreens to decrease UVR-induced pigmentation was tested. Various sunscreen formulas with quite similar SPF but with very different UVA-PF and absorption spectra were compared (Table 3). Each subject was exposed on one untreated site and on sunscreen treated sites with different doses of UV-DL, as defined in the standardized SPF testing method, taking into account the expected SPF of each product. Unprotected minimal pigmentation dose (MPDu) and protected minimal pigmentation dose (MPDp), which are the lowest UV-DL dose to produce the first pigmentation with defined borders, were assessed visually 7 days after exposure. The ratio of MPDp to MPDu gives the pigmentation protection factor (PPF). The results (Table 3) showed that, with the same level of SPF, the higher the UVA-PF, the higher the protection against pigmentation or PPF. They also demonstrated that only well-balanced products with a ratio SPF/UVA-PF < 3 are able to reduce effectively the UV-induced pigmentation.
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| OC: Octocrylene; TDSA: terephthalylidene dicamphor sulfonic acid; BMDM: butyl methoxy dibenzoyl methane; TiO2: titanium dioxide; OMC: octyl methoxycinnamate; OS: octyl salicylate; BEMT: bis-ethylhexyloxyphenol methoxyphenyl triazin; ES: ethylhexyl salicylate; DTS: drometrizole trisiloxane. | ||||||
| Absorbers composition | OC | OMC | OC | OC | OC | OMC |
| TDSA | OS | ES | ES | ES | DTS | |
| BMDM | BEMT | TDSA | DTS | TDSA | TiO2 | |
| TiO2 | DTS | BMDM | DTS | |||
| BMDM | TiO2 | BMDM | ||||
| TiO2 | TiO2 | |||||
| SPF | 19 | 19 | 30 | 30 | 50 | 50 |
| UVA-PF | 8 | 4 | 15 | 9 | 21 | 13 |
| SPF/UVA-PF | 2.4 | 4.8 | 2 | 3.3 | 2.4 | 3.8 |
| PPF | 17.2 | 11.7 | 18.9 | 9 | 58.9 | 22.3 |
Prevention of DNA damage
Reconstructed human skin in vitro,25 composed of a living dermal equivalent and a fully differentiated epidermis when exposed to UVA, induced the expression of numerous genes in fibroblasts (Fb) and keratinocytes (Kc). These genes are involved in extracellular matrix homeostasis (e.g.MMP1), oxidative stress (e.g.HO1, metallothionein 1G), heat shock response (e.g.HSP1A), cell growth (e.g.growth differentiation factor 15), inflammation (e.g.IL6, IL8, Cox2) and epidermal differentiation (e.g. TNF alpha, ODC 1). The delivered dose of UV was 30 J cm−2 of UVA (320–400 nn). When the reconstructed skin was treated before exposure with a SPF 50+, UVA-PF 30 sunscreen (containing OC, BMDM, OMC, TDSA, TiO2, BEMT, MBMT, DMTS) the modulation of most gene expression was significantly reduced or abrogated. After UVA exposure without protection, 44 out of 202 detected transcripts in Kc and 32 out of 191 transcripts in Fb were modulated (at least two-fold). These numbers were reduced to 11/202 in Kc and 4/191 in Fb when the exposure was done after the sunscreen application (2 mg cm−2). In addition, most of the genes modulated in the exposed cells protected by sunscreen were not different from those modulated in unprotected UVA-exposed cells. Many of them also showed a much lower intensity of modulation. In the same study, it was shown also that the amount of proteins secreted in the culture medium by some selected genes was increased after UVA exposure and that sunscreen inhibited this increase.An in vivo study25 conducted in parallel with the same sunscreen, on human volunteers, exposed to increasing doses of UVA-1 (340–400 nm) confirmed that UVA modulated the following genes: MMP1, HO1, GPX, CAT, SOD-2 and that the broad-spectrum sunscreen (SPF 50+, UVA-PF 30) inhibited the induction of all genes assessed.
As mentioned in the introduction, UVA radiation produces reactive oxygen species (ROS). These radicals damage DNA by inducing DNA strand breaks. These DNA breaks can be quantified in vitro using the comet assay also named single cell electrophoresis. Marrot et al.26 evaluated the protection against this DNA lesion provided by two sunscreen products A and B: SPF 7.5, UVA-PF 7 (A = 7% OC + 3% BMDM) and SPF 7.5, UVA-PF 3 (B = 3.75% OMC + 7.5% ZnO) using the comet assay with melanocytes. Fig. 1 shows the absorption spectra of these 2 products. Sunscreen products were spread on a quartz slide placed over the melanocyte culture before exposure to SSR. The measurement of tail moment showed that both sunscreens reduced the length, the number and the intensity of comets. The product with the UVA-PF of 7 was significantly more protective than the comparator containing UVA-PF 3.
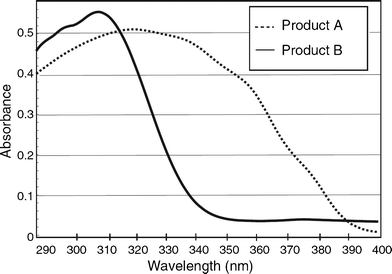 | ||
| Fig. 1 Absorption spectra of the 2 sunscreen products A (SPF 7.5 and UVA-PF 7) and B (SPF 7.5 and UVA-PF 3) evaluated. | ||
In response to a genotoxic stress, similar to such induced by UV exposure, the product of the tumor suppressor gene p53 accumulates in the epidermis. It binds to DNA and in so doing, regulates cell cycle progression by preventing repair or triggering apoptosis of the damaged cells. The accumulation of the p53 protein can be quantified and used to evaluate DNA damage and protection afforded by sunscreens. The same two SPF 7.5 sunscreen products A and B evaluated in vitro on melanocytes were compared in humans.27 These products had a UVA-PF of 7 (A) and 3 (B) respectively. Nuclear p53 protein was quantified in biopsies treated with the sunscreens and exposed 8 times to 5 MED of SSR. The results showed (Fig. 2) that both sunscreens offered only partial protection against these high repetitive exposures, but there was significantly less p53 positive nuclei in the epidermis of the skin treated with the UVA-PF 7 product A (Fig. 2-B) as compared with the low UVA-PF product B (Fig. 2-C).27
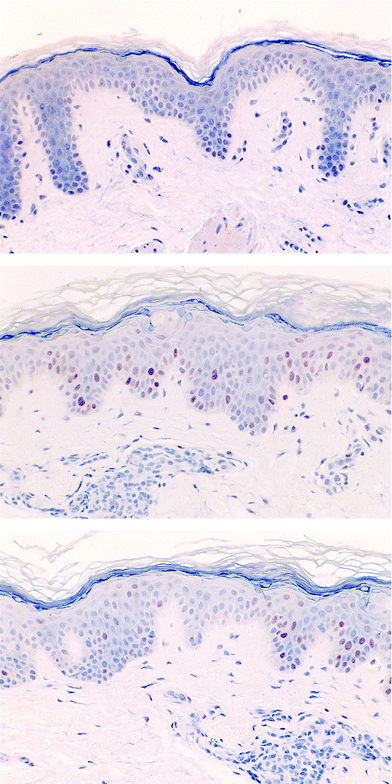 | ||
| Fig. 2 p53 immunoperoxidase detection in unexposed control skin (A) or in skin exposed to SSR (5 individual MED repeated 8 times over the course of 2 weeks)27 after application of either sunscreen product A with UVA-PF of 7 (B) or B with a UVA-PF of 3 (C) (see Fig. 1). Skin sections were stained with antibody against p53 protein.27 | ||
Protection of the immune system
Until recently, little was known about the wavelength dependency of UV-induced immunosuppression. This was particularly true for UVA radiation. There has even been controversy over whether UVA was immune suppressive or immune protective.28–30 Matthews et al.,31 using the nickel model of recall contact hypersensitivity (CHS), showed that UVA and particularly long wavelengths (364–385 nm) were immunosuppressive. This was also demonstrated with total UVA (320–400 nm)32,33 using the elicitation of delayed type hypersensitivity (DTH) reaction to recall antigens.The ability of sunscreen to reduce or inhibit UVR-induced suppression of either the induction arm or the elicitation arm of the CHS reaction as well as the elicitation of DTH response has been widely studied34–38 following acute or repeated doses of SSR. All these studies reported that acute or repeated sub-erythemal doses induced immunosupression. They also demonstrated that immune protection, provided by sunscreens, is lower than the protection against erythema, and that broad-spectrum sunscreens containing both UVB and UVA filters are significantly more protective than UVB filters. In addition, it appears that the higher the UVA protection level, the higher the immune protection.
Prevention of photoaging
Photoaging is induced by repeated solar UV exposure. If sunscreen reduces the number of photons reaching the living layers of the epidermis and dermis, it would be effective in reducing this long-term damage.In the literature, there are only a small number of studies of sunscreen efficacy against photoaging or markers of photoaging in humans.39–42 Most investigators used a solar simulator that simulates the sun at its zenith in summer with a clear sky as a UVR source. As the emitted spectra contained a maximal proportion of UVB radiation, the biological damage arising under these conditions was mostly UVB related. However, it is difficult to highlight the role of UVA filters in sunscreens. Furthermore, this spectrum is not representative of the spectrum most people receive during their life while undertaking usual outdoor activities. A more relevant source would be UV-daylight (UV-DL),22 which has a UVA to UVB irradiance ratio of 25, instead of 10 for the classical SSR sources. Using this UV source, it has been demonstrated43 that repeated exposure to sub-erythemal doses of UV-DL induces damage in the epidermis and the dermis resembling that observed in photoaging. Nineteen daily doses of 0.5 MED induced increases in epidermal thickness, number of p53 positive cells, lysozyme to elastin deposition, and number and size of DOPA+ melanocytes. It also decreased the number of Langerhans cells and glycoaminoglycan deposition.
In a further study, Seite et al.44 reported that a low-SPF well-balanced daily care product (SPF 8, UVA-PF 7) containing BMDM and TDSA as UVA filters and OC as UVB filter, inhibited most of the effects induced by the same regimen of UV-DL exposure. Only the epidermal thickening and proliferation were not reduced.
With this UVR source (UV-DL) it was also demonstrated in vitro, in a reconstructed skin model composed of a living dermal equivalent and a fully differentiated epidermis, that UV-DL induced the disappearance of dermal fibroblasts and an increase in matrix metalloprotease-1 production in the culture medium.45 The efficacy against these biological changes of two sunscreens with a similar SPF of 15 having a critical wavelength higher than 370 nm but different levels of UVA-PF (10.4 versus 2.4) was compared. Sunscreen A contained BMDM, TDSA and OC, and sunscreen B contained OMC and ZnO. Another pair of sunscreens was also tested: sunscreen C with a SPF of 18 with a well balanced UVB-UVA absorption (TDSA, BMDM, DMTS, OC), and sunscreen D with a SPF of 27 but with low UVA absorption (OC, OMC, ethylhexyl salicylate (ET, ethylhexyl triazone or octisalate), ZnO). The results showed that products A and C with the high UVA absorption gave better protection than products B and D.45 For example, the protection of dermal fibroblasts by sunscreen A and B as presented in Fig. 3.
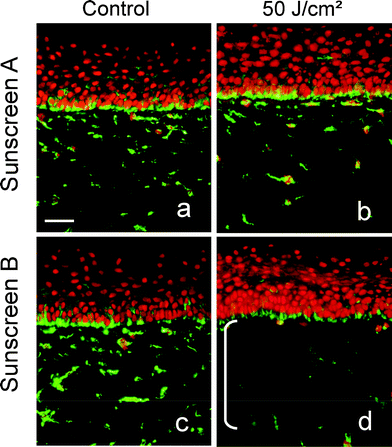 | ||
| Fig. 3 Protection of dermal fibroblasts by sunscreen A (SPF 7.5 and UVA-PF 7) and B (SPF 7.5 and UVA-PF 3) (see Fig. 1) using vimentin immunostaining made 48 h after UV-DL esposure (0 or 50 J cm−2) on reconstructed skin where product A (a, b) or product B (c, d) was applied before exposure.45 | ||
These in vivo and in vitro studies demonstrate that sunscreens with well-balanced UVB and UVA protection (SPF/UVAPF < 3) even at a low SPF are able to reduce biological cutaneous changes that may lead to photoaging.
Prevention of photodermatoses
UVA radiation often causes photodermatoses even though some of them are induced by UVB or visible light or by a combination of all wavelengths. For this reason, broad-spectrum sunscreens with high SPF and high UVA-PF provide an efficacious protection for the majority of patients. The most studied photodermatoses are the polymorphic light eruption (PMLE) and the lupus erythematosus (LE).In a study reported in 2008,46PMLE patients were exposed daily to real sunlight for 7 consecutive days. They applied per half-body two different SPF 50+ sunscreens with very different UVA-PF (4 versus 28). Fifteen of the 16 volunteers experienced eruptions on the body site protected by the low UVA-PF photo-unstable sunscreen compared to 4 on the body sites treated with the high UVA-PF photostable product
In another study, 10 females prone to PMLE were sun-exposed during 6 days after applying two products, one per half-body. With a SPF 50+, UVA PF 28 (ratio SPF/UVAPF 2.1) sunscreen A, only 3 delayed eruptions were observed when 9 cases of PMLE appeared on the body sites treated with a SPF 50+, UVAPF 17 (ratio SPF/UVAPF 3.5) sunscreen B (Fig. 4). Both products have a critical wavelength value higher than 370 nm, however product A has the best UVA/UVB protection balance and thus has a better efficacy compared to product B. These results (unpublished data) show again the importance of a high level UVA protection.
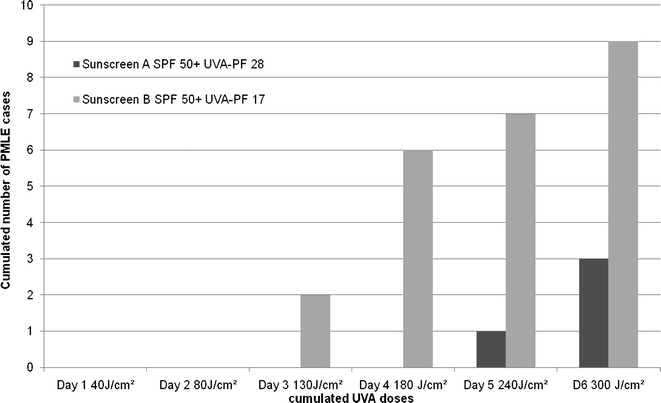 | ||
| Fig. 4 Protection of PMLE-prone subjects by either product A (SPF/UVA-PF = 2.1) or B (SPF/UVA-PF = 3.5). | ||
In 2000, Stege et al.47 reported the efficacy of three sunscreens to prevent UV-induced generation of skin lesions in eleven LE patients. A standard provocative phototest protocol was used with a source delivering UVA and UVB radiation. All the 11 patients developed LE-specific skin lesions on the unprotected site. The most effective sunscreen was the high SPF, high UVA-PF sunscreen (OC, TDSA, DMTS, BMDM, TiO2) which protected all patients. Five patients were protected by the high (similar) SPF, medium UVA-PF sunscreen (OMC, OT, Neohelipan, 4-MBC, BMDM, TiO2) and only three patients were free of lesions on the site treated by the low SPF, low UVA-PF product (4-MBC, OMC, OT, BMDM, TiO2). From these results and those of Kuhn et al.,48 it is assumed that to effectively protect photosensitive patients with LE from developing skin lesions the use of sunscreen with broad absorption spectra, a high SPF and a high UVA-PF is suggested.
Conclusion
Over the past twenty years, an increasing number of publications have reported the damaging effects of UVA radiation. It has been proven that UVA radiation induces molecular, cellular and clinical damage, which may lead to photoaging, immune system depression, altered gene expression, oncogenes and tumor suppressor gene modulation partly responsible for skin cancer development. In parallel to this increased knowledge, progress has been achieved in sunscreen technology. A variety of UVA filters have been developed. Formulators combined them with UVB filters in order to reach high photostable protection with a minimum concentration of active ingredients. However, there remains a need for harmonization of testing methods and labelling of UVA protection in sunscreens and for approval of new filters, particularly in the US.In this article, we demonstrated that the protection against UV-induced skin damage provided by sunscreen products with the same SPF but different UVA-PF is markedly different, emphasising the importance of high UVA protection in preventing cell damage. Only well-balanced (ratio SPF/UVAPF < 3), photostable sunscreens absorbing over the entire UV spectrum of sun radiation are able to maintain, unaltered, the essential biological functions.
Further demonstration, in real life conditions, of the prevention of long-term effects, such as photoaging and skin cancer, has to be done. Particularly in the light of findings reported recently by A. Green et al.:49,50 a sunscreen factor SPF 16 with some UVA protection (2% BMDM) applied daily for 4.5 years by an Australian population was able to reduce the incidence of squamous cell carcinoma. It was also demonstrated that 10 years after trial cessation, this sunscreen decreased the risk and the severity of cutaneous melanoma.
This last result may be a convincing argument to encourage consumers to use sun protection products, in association with other means of protection such as shade and a hat, on a regular basis. Modern sunscreen formulations with increased UVA protection level and well-balanced SPF/UVAPF ratio have to be recommended.
Abbreviations
| BEMT | Bis-ethylhexyloxyphenol methoxyphenyl triazin |
| BMDM | Butyl methoxy dibenzoyl methane |
| DHHB | Diethylamino hydroxybenzoyl hexyl benzoate |
| DPDT | Disodium phenyl dibenzimidazole tetrasulfonate |
| DTS | Drometrizole trisiloxane |
| ES | Ethylhexyl salicylate |
| ET | Ethylhexyl triazon |
| MBBT | Methylene bis-benzotriazolyl tetramethylbutylphenol |
| 4-MBC | 4-Methylbenzylidene camphor |
| OC | Octocrylene |
| OMC | Octyl methoxycinnamate |
| OS | Octyl salicylate |
| TDSA | Terephthalylidene dicamphor sulfonic acid |
| TiO2 | Titanium dioxide |
| ZnO | Zinc oxide |
| ROS | Reactive oxygen species |
| SPF | Sun protection factor |
| UVA-PF | UVA protection factor |
| OTC | Over-the-counter |
| NDA | New drug application |
| TEA | Time and extent application |
| UVR | UV radiation |
| SSR | Solar simulated radiation |
| EC | European Commission |
| FDA | Food and Drug Administration |
| PPD | Persistent pigment darkening |
| ISO | International Standardisation Organisation |
| COLIPA | European Cosmetic Industry Association |
| UV-DL | UV daylight |
| MED | Minimal erythema doses |
| MPDu | Unprotected minimal pigmentation dose |
| MPDp | Protected minimal pigmentation dose |
| PPF | Pigmentation protection factor |
| Fb | Fibroblasts |
| Kc | Keratinocytes |
| CHS | Contact hypersensitivity |
| DTH | Delayed type hypersensitivity |
| PMLE | Polymorphic light eruption |
| LE | Lupus erythematosus |
| INCI | International Nomenclature of Cosmetic Ingredients |
References
- D. Moyal and A. Fourtanier, Acute and chronic effects of UV on skin, in Photoaging, ed. D. S. Rigel, R. A. Weiss, H. W. Lim and J. S. Dover, NewYork, Marcel Dekker, Inc, 2004, pp. 15–32 Search PubMed.
- W. A. Bruls, H. Slaper, J. C. Van der Leun and L. Berrens, Transmission of human epidermis and stratum corneum as a function of thickness in the ultraviolet and visible wavelengths, Photochem. Photobiol., 1984, 40, 485–494 CrossRef CAS.
- K. H. Kaidbey and A. M. Kligman, The acute effects of long wave ultraviolet light upon human skin, J. Invest. Dermatol., 1979, 72, 253–256 CAS.
- A. D. Pearse, S. A. Gaskell and R. L. Marks, Epidermal changes in human skin following irradiation with either UVB or UVA, J. Invest. Dermatol., 1987, 88, 83–87 CAS.
- S. Mouret, M. T. Leccia, J. L. Bourrain, T. Douki and J. C. Beani, J. Invest. Dermatol., 2011, 131, 1539–1546 CrossRef CAS.
- J. Kuchel, R. Barnetson and G. Halliday, Ultraviolet A augments solar simulated ultraviolet radiation-induced local suppression of recall responses in humans, J. Invest. Dermatol., 2002, 118, 1032–1037 CrossRef CAS.
- R. Lavker and K. Kaidbey, The spectral dependence for UVA-induced cumulative damage in human skin, J. Invest. Dermatol., 1997, 108, 17–21 CAS.
- C. F. Garland, F. C. Garland and E. C. Gorham, Epidemiologic evidence for different roles of ultraviolet A and B radiation in melanoma mortality rates, Ann. Epidemiol., 2003, 13, 395–404 CrossRef.
- D. Lazovich, R. I. Vogel, M. Berwick, M. A. Weinstock, K. E. Anderson and E. M. Warshaw, Indoor tanning and risk of melanoma: a case-control study in a highly exposed population, Cancer Epidemiol., Biomarkers Prev., 2010, 19, 1557–1568 CrossRef.
- K. Hasei and M. Ichihashi, Solar urticaria: determinations of action and inhibition spectra Arch, Arch. Dermatol., 1982, 118, 346–350 CAS.
- B. Ortel, A. Tanew, K. Wolff and H. Honisgman, Polymorphous light eruption: action spectrum and photoprotection, J. Am. Acad. Dermatol., 1986, 14, 748–753 CrossRef CAS.
- E. Gonzalez and S. Gonzalez, Drug photosensitivity: idiopathic photodermatoses and sunscreens, J. Am. Acad. Dermatol., 1996, 35, 871–887 CrossRef CAS.
- S. Seité, A. Fourtanier, D. Moyal and A. Young, Photodamage to human skin by suberythemal exposure to solar ultraviolet radiation can be attenuated by sunscreens: a review, Br. J. Dermatol., 2010, 163, 903–914 CrossRef.
- Council Directive 76/768/EEC, Annex VII List of UV filters which cosmetic products may contain.
- Federal Register, Food and Drug Administration (USA) Department of Health and Human Services, Sunscreen Drug Products for Over-the-counter Human Use, 1999, No 64, p 27666–27693.
- European Commission, On the efficacy of sunscreens products and the claims made relating thereto, Off. J. Eur. Union, 2006, 265, 39–43 Search PubMed.
- Federal Register, Food and Drug administration (USA) Department of Health and Human Services, Labeling and Effectiveness Testing: Sunscreen Drug Products for Over-the-counter Human Use, 2011, No 21, p 201 and 310.
- JCIA, Measurements standard for UVA protection efficacy, Jan.1, 1996.
- COLIPA guidelines 2007, Method for in vitro determination of UVA protection provided by sunscreen products, COLIPA 2007, Brussels.
- COLIPA guidelines 2011, In vitro method for the determination of the UVA protection factor and “critical wavelength” values of sunscreen products, COLIPA 2011, Brussels.
- A. R. Young, J. Boles, B. Herzog, U. Osterwalder and W. Baschong, A sunscreen's labelled sun protection factor may overestimate protection at temperate latitudes: a human in vivo study, J. Invest. Dermatol., 2010, 130, 2457–2462 CrossRef CAS.
- F. J. Christiaens, A. Chardon, A. Fourtanier and J. Frederick, Standard ultraviolet daylight for non extreme exposure conditions, Photochem. Photobiol., 2005, 81, 874–878 CrossRef CAS.
- Y. Miyamura, S. G. Coelho, K. Schlenz, J. Batzer, C. Smuda, W. Choi, M. Brenner, T. Passeron, G. Zhang, L. Kolbe, R. Wolber and V. J. Hearing, The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin, Pigm. Cell Melanoma Res., 2011, 24, 136–147 CrossRef.
- D. Moyal, Prevention of pigmentation in Asian skin exposed to ultraviolet daylight, Proceeding of the International Federation of the Societies of Cosmetic Chemists (IFSCC) meeting Osaka, Japan, October 2006.
- C. Marionnet, S. Grether-Beck, S. Seité, A. Marini, T. Jaenicke, F. Lejeune, P. Bastien, A. Rougier, F. Bernerd and J. Krutmann, A broad-spectrum sunscreen prevents UVA radiation-induced gene expression in reconstructed skin in vitro and in human skin in vivo, Exp. Dermatol., 2011, 20(6), 477–482 CrossRef CAS.
- L. Marrot, J. P. Belaidi and J. R. Meunier, The human melanocyte as a particular target for UVA radiation and an endpoint for photoprotection assessment, Photochem. Photobiol., 1999, 69, 686–693 CrossRef CAS.
- S. Seité, D. Moyal, M. P. Verdier, C. Hourseau and A. Fourtanier, Accumulated p53 Protein and UVA protection level of sunscreens, Photodermatol., Photoimmunol. Photomed., 2000, 16, 3–9 Search PubMed.
- V. E. Reeve, M. Bosnic, C. Boehm-Wilcox, N. Nishimura and R. D. Ley, Ultraviolet A radiation (320–400 nm) protects hairless mice from immunosuppression induced by ultraviolet B radiation (280–320 nm) or cis-urocanic acid, Int. Arch. Allergy Immunol., 1998, 115, 316–322 CrossRef CAS.
- V. E. Reeve and R. M. Tyrrell, Heme oxygenase induction mediates the photoimmunoprotective activity of UVA radiation in the mouse, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 9317–21 CrossRef CAS.
- J. Garssen, F. de Gruijl, D. Mol, A. de Klerk, P. Roholl and H. Van Loveren, UVA exposure affects UVB and cis-urocanic acid-induced systemic suppression of immune responses in Listeria monocytogenes-infected Balb/c mice, Photochem. Photobiol., 2001, 73, 432–428 CrossRef CAS.
- Y. J. Matthews, G. M. Halliday, T. A. Phan and D. L. Damian, Wavelength dependency for UVA-induced suppression of recall immunity in humans, J. Dermatol. Sci., 2010, 59, 192–197 CrossRef CAS.
- D. Moyal and A. Fourtanier, Broad-spectrum sunscreens provide better protection from suppression of the elicitation phase of delayed-type hypersensitivity response in humans, J. Invest. Dermatol., 2001, 117, 1186–1192 CrossRef CAS.
- D. Moyal and A. Fourtanier, Effects of UVA radiation on an established immune response in humans and sunscreen efficacy, Exp. Dermatol., 2002, 11(s1), 28–32 CrossRef CAS.
- P. Wolf, C. Hoffmann, F. Quehenberger, S. Grinschgl and H. Kerl, Immune protection factors of chemical sunscreens measured in the local contact hypersensitivity model in humans, J. Invest. Dermatol., 2003, 121, 1080–1087 CrossRef CAS.
- E. D. Baron, A. Fourtanier, D. Compan, C. Medaisko, K. D. Cooper and S. R. Stevens, High ultraviolet a protection affords greater immune protection confirming that ultraviolet A contributes to photoimmunosuppression in humans, J. Invest. Dermatol., 2003, 121, 869–875 CrossRef CAS.
- T. S. Poon, R. S. Barnetson and G. M. Halliday, Prevention of immunosuppression by sunscreens in humans is unrelated to protection from erythema and dependent on protection from ultraviolet a in the face of constant ultraviolet B protection, J. Invest. Dermatol., 2003, 121, 184–190 CrossRef CAS.
- D. A. Kelly, P. T. Seed, A. R. Young and S. L. Walker, A commercial sunscreen's protection against ultraviolet radiation-induced immunosuppression is more than 50% lower than protection against sunburn in humans, J. Invest. Dermatol., 2003, 120, 65–71 CrossRef CAS.
- D. Moyal and A. M. Fourtanier, Efficacy of broad-spectrum sunscreens against the suppression of elicitation of delayed-type hypersensitivity responses in humans depends on the level of ultraviolet A protection, Exp. Dermatol., 2003, 12, 153–159 CrossRef CAS.
- R. M. Lavker, G. F. Gerberick, D. Veres, C. J. Irwin and K. H. Kaidbey, Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin, J. Am. Acad. Dermatol., 1995, 32, 53–62 CrossRef CAS.
- A. R. Young, G. E. Orchard, G. I. Harrison and J. L. Klock, The detrimental effects of daily sub-erythemal exposure on human skin in vivo can be prevented by a daily-care broad-spectrum sunscreen, J. Invest. Dermatol., 2006, 127(4), 975–978 CrossRef.
- S. Seité, A. Colige, P. Piquemal-Vivenot, C. Montastier, A. Fourtanier, C. Lapière and B. Nusgens, Photodermatol., Photoimmunol. Photomed., 2000, 16, 147–55 Search PubMed.
- S. Seité and A. M. Fourtanier, The benefit of daily photoprotection, J. Am. Acad. Dermatol., 2008, 58, S160–166 CrossRef.
- S. Seité, C. Medaisko, F. Christiaens, C. Bredoux, D. Compan, H. Zucchi, D. Lombard and A. Fourtanier, Biological effects of simulated ultraviolet daylight: a new approach to investigate daily photoprotection, Photodermatol., Photoimmunol. Photomed., 2006, 22, 67–77 CrossRef.
- S. Seité, F. Christiaens, C. Bredoux, D. Compan, H. Zucchi, D. Lombard, A. Fourtanier and A. R. Young, A broad-spectrum sunscreen prevents cumulative damage from repeated exposure to sub-erythemal solar ultraviolet radiation representative of temperate latitudes, J. Eur. Acad. Dermatol. Venereol., 2010, 24, 219–222 CrossRef.
- F. Lejeune, F. Christiaens and F. Bernerd, Evaluation of sunscreen products using a reconstructed skin model exposed to simulated daily ultraviolet radiation: relevance of filtration profile and SPF value for daily photoprotection, Photodermatol., Photoimmunol. Photomed., 2008, 24, 249–255 CrossRef.
- A. Fourtanier, D. Moyal and S. Seité, Sunscreens containing the broad-spectrum UVA absorber, Mexoryl SX, prevent the cutaneous detrimental effects of UV exposure: a review of clinical study results, Photodermatol., Photoimmunol. Photomed., 2008, 24, 164–174 CrossRef CAS.
- H. Stege, M. A. Budde, S. Grether-Beck and J. Krutmann, Evaluation of the capacity of sunscreens to photoprotect lupus erythematosus patients by employing the photoprovocation test, Photodermatol., Photoimmunol. Photomed., 2000, 16, 256–259 CAS.
- A. Kuhn, K. Gensch, M. Haust, A. M. Meuth, F. Boyer, P. Dupuy, P. Lehmann, D. Metze and T. Ruzicka, Photoprotective effects of a broad-spectrum sunscreen in ultraviolet-induced cutaneous lupus erythematosus: a randomized, vehicle-controlled, double-blind study, J. Am. Acad. Dermatol., 2011, 64, 37–48 CrossRef CAS.
- A. Green, G. Williams, R. Neale, V. Hart, D. Leslie, P. Parsons, G. C. Marks, P. Gaffney, D. Battistutta, C. Frost, C. Lang and A. Russell, Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial, Lancet, 1999, 354, 723–729 CrossRef CAS.
- A. C. Green, G. M. Williams, V. Logan and G. M. Strutton, Reduced melanoma after regular sunscreen use: randomized trial follow-up, J. Clin. Oncol., 2010, 29, 257–263 CrossRef.
Footnotes |
| † Contribution to the themed issue on the biology of UVA. |
| ‡ Funding: SS, DM and AF are employees of L'Oréal, France. Conflict of interest statement: All authors are employees of L'Oréal |
| This journal is © The Royal Society of Chemistry and Owner Societies 2012 |
