 Open Access Article
Open Access ArticleHypoiodous acid initiated rearrangement of tertiary propargylic alcohols to α-iodoenones†
Wesley J.
Moran
* and
Arantxa
Rodríguez
*
Department of Chemical and Biological Sciences, University of Huddersfield, Huddersfield HD1 3DH, UK. E-mail: w.j.moran@hud.ac.uk; a.r.menendez@hud.ac.uk; Fax: +44 (0)1484 472182; Tel: +44 (0)1484 473741
First published on 3rd October 2012
Abstract
In the presence of an oxidant, sodium iodide is converted into hypoiodous acid which effects the rearrangement of tertiary propargylic alcohols to α-iodoenones in good yields.
Polyvalent iodine species are mild and selective reagents that have attracted significant attention in organic synthesis because of their range of useful reactivity, low toxicity, and ease of use and handling.1 The majority of research has concentrated on the use of iodine(III) and iodine(V) compounds, however very recently iodite(I) and iodite(III) species have emerged as useful oxidants in a handful of reactions.2
Substituted propargylic alcohols are readily prepared from aldehydes/ketones and terminal alkynes and serve as versatile synthetic intermediates.3 They have been utilized as starting materials in a variety of processes including polyvalent iodine mediated transformations such as the NIS mediated rearrangement/iodination of acyclic tertiary propargylic alcohols (e.g.1),4 and the polymer supported iodobenzene diacetate mediated ring expansion/iodination of 1-alkynylcycloalkanols (e.g.2a) to β-iodoenones 3 (Scheme 1).5 Both of these reactions involve carbonyl formation as the driving force in 1,2-rearrangement processes.
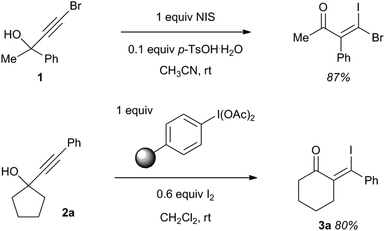 | ||
| Scheme 1 Polyvalent iodine mediated rearrangements of tertiary propargylic alcohols. | ||
Bovonsombat and McNelis showed that the three tertiary propargylic alcohols 2a–c could be rearranged to α-iodoenones 4a–c in moderate yields using one equivalent of NIS and 0.1 equivalents of Koser's reagent (PhI(OH)OTs) in methanol (Scheme 2).6 Little preference for either alkene isomer was observed. Intriguingly, under these conditions the oxygen is the migrating group. Zhang and co-workers reported a related gold-catalyzed rearrangement of propargylic acetates to α-iodoenones.7
 | ||
| Scheme 2 NIS mediated rearrangement. | ||
As part of a wider program to investigate the reactivity of polyvalent iodine species, we were interested in developing rearrangements of tertiary propargylic alcohols such as 2 utilizing hypoiodite species. It is known that treating iodide with oxidants can lead to the formation of hypoiodous acid (or iodic acid), however its reactivity has been little studied.2 At the start of our investigation with cyclic tertiary propargylic alcohols 2, four possible outcomes were identified: (i) no reaction; (ii) rearrangement with ring expansion; (iii) rearrangement without ring expansion; or (iv) a new reaction.
In the event, alcohol 2d was treated with m-CPBA, p-toluenesulfonic acid and sodium iodide in acetonitrile at room temperature (Table 1, entry 1). Perhaps unsurprisingly, the strong acid led to dehydration of the starting material and enyne 5 was isolated in 83% yield. Presumably, the alkene was not epoxidised as the m-CPBA had been used up in oxidising the iodide. Changing the acid to trifluoroacetic acid also led to isolation of enyne 5 in 80% yield (entry 2). With acetic acid, rearrangement occurred, without ring expansion, to provide α-iodoenone 4d but in only 9% yield (entry 3).8 Changing to trichloroacetic acid, the α-iodoenone 4d was formed in 50% yield (entry 4). Exchanging sodium iodide for tetrabutylammonium iodide led to a similar yield of product however the 1H NMR spectrum of the crude reaction mixture was noticeably messier, therefore the use of sodium iodide was preferred (entry 5). The amount of oxidant was increased to three equivalents and 75% of the α-iodoenone 3 was isolated (entry 6). The reaction was carried out without the acid which led to product formation, however in just 8% yield (entry 7). None of the ring expanded product 3d was evident in any of these reactions. Trichloroacetic acid is envisaged to have just the right acidity to lead to formation of 4 without causing formation of enyne 5. The use of hydrogen peroxide in place of m-CPBA led to no reaction.
|
|
|||
|---|---|---|---|
| Entry | Acid | Iodide | Yielda (%) |
| a Yield of pure isolated product. b Yield determined by 1H NMR analysis. c 3 equiv. of m-CPBA used. | |||
| 1 | 4-Toluenesulfonic acid monohydrate | NaI | 83 (5) |
| 2 | Trifluoroacetic acid | NaI | 80 (5) |
| 3 | Acetic acid | NaI | 9b (4d) |
| 4 | Trichloroacetic acid | NaI | 50b (4d) |
| 5 | Trichloroacetic acid | Bu4NI | 50b (4d) |
| 6 | Trichloroacetic acid | NaI | 75c (4d) |
| 7 | — | NaI | 8b (4d) |
With the optimized conditions in hand, the scope of the rearrangement process was investigated (Scheme 3).‡ Varying the aromatic substituent had no adverse effect on the reaction outcome and the α-iodoenones were formed in good yields. Increasing the size of the ring to six, seven and eight membered and using open chain substrates also led to efficient rearrangements in all cases studied. Unsymmetrical substrate 2p (R1 = Me, R2 = Et) underwent rearrangement to provide 4p as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of separable E and Z alkene isomers, whereas 2q (R1 = Me, R2 = t-Bu) rearranged stereoselectively to 4q (only the E isomer could be identified by NMR analysis of the crude reaction mixture).9 These results can be rationalised by consideration of the steric interactions. In the former case, methyl and ethyl are of similar size whereas a tert-butyl group is much larger than a methyl substituent. The phenyl ketone group can rotate out of the plane of the alkene leaving more space for the tert-butyl. However, this result is surprising considering the NIS-mediated rearrangement of 2c to 4c only gave a 1
1 mixture of separable E and Z alkene isomers, whereas 2q (R1 = Me, R2 = t-Bu) rearranged stereoselectively to 4q (only the E isomer could be identified by NMR analysis of the crude reaction mixture).9 These results can be rationalised by consideration of the steric interactions. In the former case, methyl and ethyl are of similar size whereas a tert-butyl group is much larger than a methyl substituent. The phenyl ketone group can rotate out of the plane of the alkene leaving more space for the tert-butyl. However, this result is surprising considering the NIS-mediated rearrangement of 2c to 4c only gave a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 E/Z ratio (vide supra).
1.5 E/Z ratio (vide supra).
 | ||
| Scheme 3 Scope of rearrangement process. | ||
The use of alkyl substituted alkynes led to very low conversions (<10%) to the α-iodoenones. Whereas secondary propargyl alcohols failed to rearrange at all under these conditions.
The mechanism of this rearrangement is postulated to proceed through oxidation of the iodide to hypoiodous acid (HOI) which reacts with the alkyne to generate an iodonium cation (Scheme 4). Addition of water, followed by a proton transfer and loss of water generates the final product 4. Iodide can also be oxidised to iodic acid (HIO3), and hypoiodous acid can disproportionate into iodic acid and iodine;10 however, as the final product is an alkenyl iodide, hypoiodous acid is probably the reactive species. Reaction with iodine should lead to formation of ring-expanded product 3, however this compound is not observed.
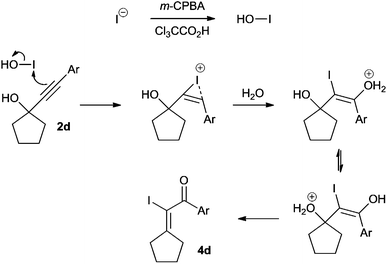 | ||
| Scheme 4 Postulated hypoiodous acid initiated rearrangement mechanism. | ||
In conclusion, we have demonstrated that in situ generated hypoiodous acid initiates the rearrangement of tertiary propargylic alcohols to α-iodoenones in good yields. This process is exceptionally simple to carry out and the products are potentially valuable for further synthesis.11 Related studies are in progress and will be reported in due course.
We thank the University of Huddersfield for funding.
Notes and references
- For reviews on hypervalent iodine chemistry, see: (a) V. V. Zhdankin, J. Org. Chem., 2011, 76, 1158 CrossRef; (b) V. V. Zhdankin, ARKIVOC, 2009, 1 CAS; (c) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2008, 108, 5299 CrossRef CAS; (d) T. Wirth, Angew. Chem., Int. Ed., 2005, 44, 3656 CrossRef CAS; (e) A. N. French, S. Bissmire and T. Wirth, Chem. Soc. Rev., 2004, 33, 354 RSC; (f) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2002, 102, 2523 CrossRef CAS; (g) G. F. Koser, Aldrichimica Acta, 2001, 34, 89 CAS; (h) P. J. Stang and V. V. Zhdankin, Chem. Rev., 1996, 96, 1123 CrossRef CAS.
- (a) A. Yoshimura, K. R. Middleton, C. Zhu, V. N. Nemykin and V. V. Zhdankin, Angew. Chem., Int. Ed., 2012, 51, 8059 CrossRef CAS; (b) Z. Liu, J. Zhang, S. Chen, E. Shi, Y. Xu and X. Wan, Angew. Chem., Int. Ed., 2012, 51, 3231 CrossRef CAS; (c) W. Wei, Y. Wang, J. Yin, J. Xue and Y. Li, Org. Lett., 2012, 14, 1158 CrossRef CAS; (d) L. Ma, X. Wang, W. Yu and B. Han, Chem. Commun., 2011, 47, 11333 RSC; (e) T. Froehr, C. P. Sindlinger, U. Kloeckner, P. Finkbeiner and B. J. Nachtsheim, Org. Lett., 2011, 13, 3754 CrossRef CAS; (f) L. Chen, E. Shi, Z. Liu, S. Chen, W. Wei, H. Li, K. Xu and X. Wan, Chem.–Eur. J., 2011, 17, 4085 CrossRef CAS; (g) A. Rodríguez and W. J. Moran, Org. Lett., 2011, 13, 2220 CrossRef; (h) M. Uyanik, H. Okamoto, T. Yasui and K. Ishihara, Science, 2010, 328, 1376 CrossRef CAS.
- For a review, see: E. B. Bauer, Synthesis, 2012, 44, 1131 CrossRef CAS.
- (a) G. J. Angara, P. Bovonsombat and E. McNelis, Tetrahedron Lett., 1992, 33, 2285 CrossRef CAS . Also, see: ; (b) P. Bovonsombat and E. McNelis, Tetrahedron, 1993, 49, 1525 CrossRef CAS.
- (a) J.-M. Chen and X. Huang, Synthesis, 2004, 15, 2459 Search PubMed . For other examples; ; (b) P. Bovonsombat and E. McNelis, Synth. Commun., 1995, 25, 1223 CrossRef CAS; (c) E. Djuardi, P. Bovonsombat and E. McNelis, Tetrahedron, 1994, 50, 11793 CrossRef CAS; (d) P. Bovonsombat and E. McNelis, Tetrahedron Lett., 1993, 34, 4277 CrossRef CAS.
- P. Bovonsombat and E. McNelis, Tetrahedron Lett., 1993, 34, 8205 CrossRef CAS.
- (a) M. Yu, G. Zhang and L. Zhang, Tetrahedron, 2009, 65, 1846 CrossRef CAS; (b) M. Yu, G. Zhang and L. Zhang, Org. Lett., 2007, 9, 2147 CrossRef CAS.
- Compounds 3d and 4d are distinguishable by 1H NMR as shown in ref. 4a and 5a.
- E-isomer assigned with the aid of NOESY experiments; see ESI† for further details.
- 5HIO → HIO3 + 2I2 + 2H2O.
- For example, see: E. Negishi, J. Organomet. Chem., 1999, 576, 179 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: General experimental methods, compound characterisation data, copies of NMR spectra. See DOI: 10.1039/c2ob26360b |
| ‡ Typical reaction procedure: Substituted propargylic alcohol 2 (0.21 mmol), m-CPBA (109 mg, 0.63 mmol), sodium iodide (31 mg, 0.21 mmol) and trichloroacetic acid (51 mg, 0.31 mmol) were dissolved in acetonitrile (1 mL) at room temperature under a nitrogen atmosphere and stirred overnight. The reaction mixture was quenched with saturated aqueous sodium thiosulfate solution and extracted with CH2Cl2. The organic layer was washed with saturated aqueous sodium bicarbonate solution, dried over MgSO4, filtered and concentrated under reduced pressure. The residue was purified by flash chromatography (silica gel, petroleum ether/ethyl acetate) to afford the corresponding α-iodoenone 4. |
| This journal is © The Royal Society of Chemistry 2012 |

