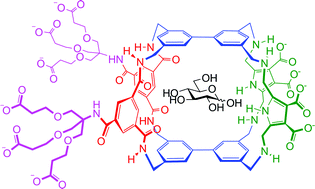
New H-bonding patterns in biphenyl-based synthetic lectins; pyrrolediamine bridges enhance glucose-selectivity†
Abstract
Synthetic lectins are molecules designed for the challenging task of biomimetic
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...