Synthesis and biological evaluation of 1,4-naphthoquinones and quinoline-5,8-diones as antimalarial and schistosomicidal agents†
Abstract
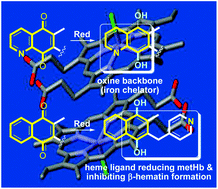
Improving the solubility of polysubstituted 1,4-naphthoquinone derivatives was achieved by introducing nitrogen in two different positions of the naphthoquinone core, at C-5 and at C-8 of menadione through a two-step, straightforward synthesis based on the regioselective hetero-Diels–Alder reaction. The antimalarial and the antischistosomal activities of these polysubstituted aza-1,4-naphthoquinone derivatives were evaluated and led to the selection of distinct compounds for antimalarial versus antischistosomal action. The AgII-assisted oxidative radical decarboxylation of the phenyl acetic acids using AgNO3 and ammonium peroxodisulfate was modified to generate the 3-picolinyl-menadione with improved pharmacokinetic parameters, high antimalarial effects and capacity to inhibit the formation of β-hematin.

 Please wait while we load your content...
Please wait while we load your content...