Combined coinage metal catalysis in natural product synthesis: total synthesis of (+)-varitriol and seven analogs†‡
Abstract
A modular total synthesis of the natural product
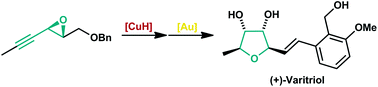
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...