Observation of neighboring ortho-hydroxyl group participation in organocatalytic asymmetric sequential Michael-lactonization reactions: synthesis of highly substituted chiral spirodihydrocoumarins†‡
Abstract
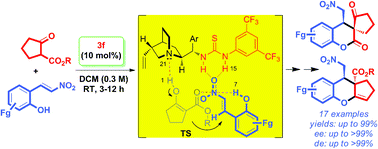
A general approach to asymmetric synthesis of highly substituted spirodihydrocoumarins with a quaternary stereocenter was achieved through neighboring ortho-hydroxyl group induced sequential Michael–lactonization reactions on 2-(2-nitrovinyl)phenols with alkyl cyclopentanone-2-carboxylates in the presence of a catalytic amount of
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...