Matthew O'Brien, Andrew Leach, Roly J. Armstrong, Keting Chong and Ross Sheridan
Org. Biomol. Chem., 2012,10, 2392-2394
DOI:
10.1039/C2OB07008A,
Communication
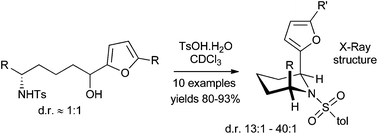
Utilising the propensity of the 2-furanyl group to facilitate equilibration of an adjacent tosylamide chiral centre, a diastereoselective route to 2,6-syn-piperidines was developed that proceeds with high levels of thermodynamic stereocontrol. X-ray crystallography structures suggest that, as seen in similar systems, pseudo-allylic strain between the N-tosyl group and the substituents at the 2 and 6 positions dominates stereochemical preference, overriding 1,3 diaxial interactions.