 Open Access Article
Open Access ArticleDiastereoselective 1,3-dipolar cycloaddition of pyrylium ylides with chiral enamides†
Kirill
Tchabanenko
*,
Colleen
Sloan
,
Yves-Mael
Bunetel
and
Philip
Mullen
School of Chemistry and Chemical Engineering, Queen's University Belfast, David Keir Building, Stranmillis Road, Belfast BT9 5AG, United Kingdom. E-mail: k.tchabanenko@qub.ac.uk
First published on 19th March 2012
Abstract
Chiral enamides 5f–i were found to react with pyrylium ylides to give cycloadducts 6d–i in good yields with an excellent level of stereoselectivity. The chiral auxiliary was successfully removed on hydrogenolysis of compound 6f in continuous flow (H-Cube) resulting in the first asymmetric synthesis of complex amine 8.
Introduction
1,3-Dipolar cycloaddition of pyrylium ylides 1 with activated alkenes is a powerful synthetic tool providing direct access to the 8-oxa-bicyclo[3,2,1]octane motif 2 (Fig. 1).1–3 Common in a number of biologically active natural products such as englerin A4,53 and aspergillin PZ,6 the oxygen bridged bicycle 2 has potential as a scaffold for the development of pharmaceuticals.![1,3-Dipolar cycloaddition approach to 8-oxa-bicyclo[3,2,1]octanes.](/image/article/2012/OB/c2ob07007c/c2ob07007c-f1.gif) | ||
| Fig. 1 1,3-Dipolar cycloaddition approach to 8-oxa-bicyclo[3,2,1]octanes. | ||
Our group became involved in finding new applications for this interesting reaction,7 with particular attention drawn to the discovery of new polarophiles and reaction conditions. Indeed, the use of nitrogen as an alkene activator will allow direct synthesis of the bicyclic amine 2 (X = NH2). Therefore it is proposed that attachment of chiral auxiliaries on the nitrogen will allow a stereoselective 1,3-dipolar cycloaddition. Only one example of a diastereoselective 1,3-dipolar cycloaddition of pyrylium ylides with chiral acrylic esters was reported by Chen and Nicolaou in their total synthesis of englerin A.5 For this study it was decided to investigate chiral enamides, which were successfully applied as polarophiles in a number of cycloaddition reactions by Hsung and co-workers.8
Results and discussion
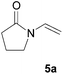
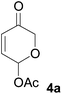
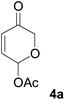
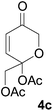
All starting materials for this study were prepared according to literature procedures. Acetoxy pyrones 4a–c were prepared from corresponding furyl alcohols9 or fructose.10 Enamides 5a, 5f and 5g were prepared via palladium catalysed trans-vinylations;11 ynamide 5e and enamides 5b–d and 5h were prepared by copper catalysed cross-couplings.12At first we decided to establish the best cycloaddition conditions for reactions with non-chiral enamides (Table 1). Acetoxy pyrone 4a was reacted under triethyl amine promoted conditions (A)2 and thermal conditions (B).1
|
|
||||
|---|---|---|---|---|
| Enamide | Conditions | Time (d) | Product | Yield |
|
A | 1.5 |
|
72% |

|
B | 4 |

|
85% |

|
A | 2 |

|
32% |

|
A | 2 | — | — |

|
A | 1.5 |

|
ca. 25% (decomposed on purification) |

|
A or B | 2 | — | — |
Under both sets of conditions the reactions with the simplest enamide 5a proceeded smoothly to give the desired product 6a. The yield was marginally better under thermal conditions, although this reaction required a significantly longer time to reach complete conversion of the starting material. The slightly lower yield of the base-promoted reaction was due to formation of the pyrylium ylide dimer 72 as a minor byproduct. Reaction of the trans-enamide 5b produced the product 6b but in a lower yield, while the cis-enamide 5c gave no detectable cycloaddition. Cycloaddition of the gem-disubstituted alkene 5d gave a different product, which decomposed upon attempts at purification by column chromatography. Our tentative assignment of the structure 6c was made on the basis of the crude 1H NMR analysis, which showed the absence of the enone double bond, and instead the presence of resonances corresponding to the double bond of the cyclic enol ether. Formation of this regioisomer is unusual and will be further investigated in a separate study.
The ynamide 5e gave no cycloaddition products under either thermal or base catalysed conditions.
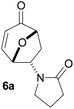
Having established that the mono-substituted double bond gave the best yields of the cycloadduct, we proceeded to investigate the reactions of chiral enamides 5f–h under base promoted milder reaction conditions (Table 2).
|
|
|||||
|---|---|---|---|---|---|
| Pyrone | Enamide | Temp | Time (h) | Product | Yield (%) |
|

|
rt | 24 |
|
59 |

|

|
rt | 48 |

|
45 |
|

|
rt | 48 |

|
51 |

|

|
50 °C | 48 |

|
63 |
|

|
rt | 24 |
|
87 |

|

|
rt | 48 |
|
21 |
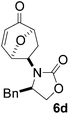
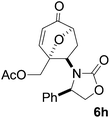
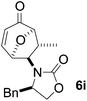
Gratifyingly, cycloaddition reactions of terminal enamides 5f–h proceeded in moderate to good yields and the products were formed as single diastereoisomers. Chiral trans-enamide 5i gave cycloadduct 6i in a lower yield as was expected from our initial experiments. The relative stereochemistry of the cycloadducts was established via X-ray analysis of 6d (Fig. 2) and further correlation of the NMR data for the products.
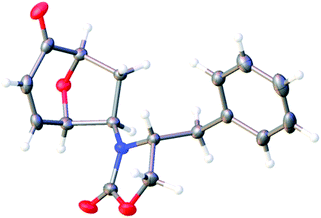 | ||
| Fig. 2 Ortep plot of the X-ray structure of 6d. | ||
The stereochemical outcome of the reactions can be rationalized by the model presented in Fig. 3. The enamide assumes the preferred conformation8 and the endo cycloaddition13 happens on the least hindered face of the alkene giving rise to the observed stereochemistry of the adducts.
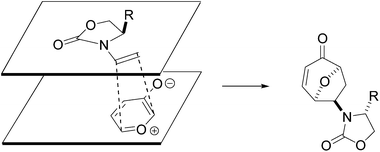 | ||
| Fig. 3 Cycloaddition model. | ||
To conclude this study we decided to demonstrate possible cleavage of the chiral auxiliary. Cycloadduct 6f was reduced on a continuous flow hydrogenation setup (H-Cube) to give both reduction of the enone double bond and cleavage of the chiral auxiliary. We discovered that direct purification of the primary amine by column chromatography was problematic due to its high polarity and the crude product was converted into an acetamide 8, which was isolated in a 50% yield after two steps (Scheme 1).
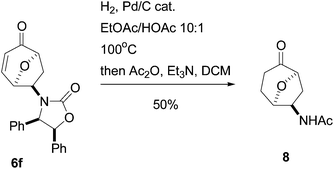 | ||
| Scheme 1 Cleavage of the chiral auxiliary. | ||
Conclusions
In summary, we have demonstrated the first example of the use of amides as alkene activators in the 1,3-dipolar cycloaddition reactions of pyrylium ylides. The use of chiral oxazolidinones as chiral auxiliaries leads to highly stereoselective cycloadditions and the first asymmetric synthesis of the bicyclic amine 8.Experimental section
All reactions were carried out under an inert atmosphere (N2) unless stated otherwise. Reaction solvents (toluene, CH2Cl2) were freshly distilled from calcium hydride under a N2 atmosphere prior to use. All NMR spectral data was collected on Bruker DRX-500 or Bruker AV-400 spectrometers. IR spectra were collected on a Perkin-Elmer Spectrum One FT-IR spectrometer, optical rotations were measured on a Perkin-Elmer Model 341 polarimeter and mass-spec data was collected on a Waters GCT-Premier spectrometer with NanoMate attachment.Base promoted cycloadditions (method A)
To a stirred solution of acetoxy pyrones 4 (1.28 mmol) and enamides 5 (6.4 mmol) in DCM (5 mL) was added triethylamine (696 μL, 5.12 mmol) in three equal portions over a period of 36 h at room temperature. Stirring was continued for another 12 h when TLC analysis indicated complete consumption of the starting material 4. Solvents and volatiles were removed under reduced pressure and the residue was purified by column chromatography (40–60 petroleum ether–ethyl acetate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the cycloadducts 6.
1) to give the cycloadducts 6.
Thermal cycloadditions (method B)
A solution of acetoxy pyrones 4 (1.28 mmol) and enamides 5 (6.4 mmol) in toluene (7 mL) was placed in a sealed tube containing a Teflon stirrer bar. The sealed tube was flushed with N2, sealed with a Teflon screw-cap and heated in an oil bath (150 °C) under stirring until TLC indicated complete consumption of the starting material 4 (usually after a period of 4 days). The reaction mixture was allowed to cool down, volatiles were removed under reduced pressure and the residue was purified by column chromatography (40–60 petroleum ether–ethyl acetate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the cycloadducts 6.
1) to give the cycloadducts 6.
Cycloadduct 6a a white solid: m.p. 76 °C; λmax(KBr) 1740 (m), 1686 (s); m/z (ES+) found 208.0974 (MH+) C11H14NO3 requires 208.0974; 1H (500 MHz, CDCl3) δ 1.82 (1H, ddd, J 14.0, 6.0, 1.5 Hz), 2.04 (2H, m), 2.40 (2H, t, J 8.5 Hz), 2.67 (1H, ddd, J 14.0, 9.0, 6.0 Hz), 3.28 (2H, m), 4.56 (1H, d, J 9.0 Hz), 4.70 (1H, dt, J 10.0, 6.0 Hz), 5.40 (1H, dd, J 6.0, 4.5 Hz), 6.15 (1H, dd, J 10.0, 1.5 Hz), 7.22 (1H, dd, J 10.0, 4.5 Hz). 13C (125 MHz, CDCl3) 0.0, 8.7, 12.6, 28.2, 36.4, 55.1, 62.1, 105.9, 132.5, 157.8, 177.9.
Cycloadduct 6b a white solid: m.p. 90–91 °C; λmax(KBr) 1752 (s), 1697 (m); m/z (ES+) found 221.1121 (MH+) C12H16NO3 requires 222.1130; 1H (500 MHz, CDCl3) δ 1.38 (3H, d, J 6.0 Hz), 2.01 (2H, m), 2.27 (1H, m), 2.41 (2H, t, J 9.0), 3.29 (2H, m), 4.13 (1H, s), 4.23 (1H, app t, J 6.0 Hz), 5.13 (1H, dd, J 6.0, 5.0 Hz), 6.11 (1H, dd, J 10.0, 1.5 Hz), 7.21 (1H, dd, J 10.0, 5.0 Hz). 13C (125 MHz, CDCl3) 0.0, 0.9, 12.6, 17.5, 28.2, 44.7, 56.3, 69.2, 109.2, 132.5, 157.8, 177.5.
Cycloadduct 6d a white solid: m.p. 131 °C; [α]20D = +370.1 (c = 0.95, CHCl3); λmax(KBr) 1750 (s), 1696 (s); m/z (ES+) found 300.1236 (MH+) C17H18NO4 requires 300.1236; 1H (500 MHz, CDCl3) δ 1.80 (1H, dd, J 13.5, 1.5 Hz), 2.70 (2H, m), 3.10 (1H, dd, J 13.5, 4.0 Hz), 3.88 (1H, ddd, J 14.0, 9.0, 8.5 Hz), 4.09 (1H, app s), 4.10 (1H, app s), 4.20 (1H, ddd, J 10.0, 6.0, 4.5 Hz), 4.50 (1H, dd, J 8.5, 1.5 Hz), 5.50 (1H, dd, J 6.0, 4.5 Hz), 6.10 (1H, dd, J 10.0, 1.5 Hz), 7.10 (1H, d, J 10.0 Hz), 7.3 (5H, m); 13C (125 MHz, CDCl3) 29.7, 37.9, 55.7, 58.9, 66.6; 74.9, 80.2, 127.5, 127.7, 129.1, 129.2, 135.1, 151.1, 157.7, 196.4.
Cycloadduct 6e a white solid; m.p. 168–170 °C; [α]20D = +8.8 (c = 1.0, CHCl3); λmax(KBr) 1755 (s), 1708 (s), 1409 (s); m/z (ES+) found 286.1089 (MH+) C16H16NO4 requires 286.1079; 1H (400 MHz, CDCl3) δ 1.68 (1H, dd, J 13.5, 1.5 Hz), 2.28 (1H, ddd, J 13.5, 10.0, 8.5 Hz), 4.02 (1H, ddd, J 10.0, 8.5, 4.5 Hz), 4.21 (1H, dd, J 8.5, 3.0 Hz), 4.38 (1H, dd, J 8.0, 1.5 Hz), 4.61 (1H, dd, J 8.5, 8.5 Hz), 4.68 (1H, dd, J 8.5, 3.0 Hz), 5.36 (1H, dd, J 6.0, 4.5), 6.17 (1H, dd, J 10.0, 1.5 Hz), 7.20–7.45 (6H, m). 13C (125 MHz, CDCl3) 29.3, 56.0, 61.5, 70.4, 74.5, 80.1, 126.2, 127.9, 129.5, 129.7, 138.3, 150.9, 158.3, 190.4.
Cycloadduct 6f a white solid; m.p. 195–199 °C; [α]20D = +25.9 (c = 2.8, CHCl3); λmax(KBr) 1742 (s), 1685 (s), 1400 (s); m/z (ES+) found 384.1230 (MNa+) C22H19NO4Na requires 384.1212; 1H (400 MHz, CDCl3) δ 1.65 (1H, dd, J 13.5, 2.0 MHz), 2.26 (1H, ddd, J 13.5, 9.5, 8.5 Hz), 4.20 (1H, ddd, J 10.0, 9.5, 6.0 Hz), 4.40 (1H, dd, J 8.5, 1.5 Hz), 4.86 (1H, d, J 7.0 Hz), 5.48 (1H, dd, J 6.0, 4.5 Hz), 5.82 (1H, d, J 7.0 Hz), 6.23 (1H, dd, J 10.0, 1.5 Hz), 5.95–7.16 (10H, m), 7.46 (1H, dd, J 10.0, 4.5 Hz). 13C (125 MHz, CDCl3) 12.1, 38.8, 49.6, 57.1, 62.7, 63.0, 108.3, 110.3, 110.5, 110.6, 111.2, 11.3, 115.7, 115.8, 139.5, 178.8.
Cycloadduct 6g a white solid; m.p. 136–138 °C; [α]20D = −267 (c = 0.4, CHCl3); λmax(KBr) 1755 (s), 1721 (m); m/z (ES+) found 336.1221 (MNa+) C18H19NO4Na requires 336.1212; 1H (500 MHz, CDCl3) δ 1.51 (3H, s), 1.98 (1H, dd, J 12.0, 6.5 Hz), 2.26 (1H, dd, J 12.0, 9.0 Hz), 2.70 (1H, dd, J 13.5, 10.0 Hz), 3.06 (1H, dd, J 13.5, 4.0 Hz), 3.84 (1H, app t, J 5.0 Hz), 4.10 (2H, app d, J 5.0 Hz), 4.32 (1H, ddd, J 9.0, 6.5, 6.0 Hz), 6.41 (1H, dd, J 6.0, 5.0 Hz), 6.13 (1H, d, J 9.5 Hz), 7.10–7.33 (6H, m). 13C (125 MHz, CDCl3) 18.5, 35.2, 36.8, 55.0, 57.7, 64.5, 74.5, 83.0, 126.4, 128.1, 128.2, 134.1, 149.8, 156.6, 196.8.
Cycloadduct 6h a white solid; m.p. 138–140 °C; [α]20D = +110.5 (c = 1.0, CHCl3); λmax(KBr) 1776 (m), 1720 (s), 1680 (m), 1257 (s); 1240 (s); m/z (ES+) found 380.1068 (MNa+) C19H19NO6Na requires 380.1110; 1H (500 MHz, CDCl3) δ 1.55 (1H, ddd, J 13.5, 6.0, 1.5 Hz), 2.11 (3H, s), 2.36 (1H, ddd, J 13.5, 10.0, 8.5 Hz), 4.10 (1H, dd, J 8.5, 2.5 Hz), 4.34 (1H, d, J 13.0 Hz), 4.40 (1H, dd, J 8.5, 1.5 Hz), 4.54 (1H, app t, J 8.5 Hz), 4.61 (1H, dd, J 10.0, 6.0 Hz), 4.65 (1H, dd, J 8.5, 2.5 Hz), 4.72 (1H, d, J 13.0 Hz), 6.30 (1H, dd, J 10.0, 1.5 Hz), 7.10–7.40 (6H, m). 13C (125 MHz, CDCl3) 3.11, 11.2, 39.9, 42.0, 47.4, 52.9, 62.4, 64.8, 108.0, 111.7, 111.9, 115.5, 121.9, 132.1, 140.6, 152.8, 177.6.
Cycloadduct 6i a pale grey viscous oil; [α]20D = −220 (c = 0.3, CHCl3); λmax(thin film) 1748 (s), 1693 (s), 1412 (m), 736 (m); m/z (ES+) found 314.1366 (MH+) C18H20NO4 requires 314.1392; 1H (500 MHz, CDCl3) δ 1.55 (1H, ddd, J 13.5, 6.0, 1.5 Hz), 2.11 (3H, s), 2.36 (1H, ddd, J 13.5, 10.0, 8.5 Hz), 4.10 (1H, dd 1H (500 MHz, CDCl3) δ 1.52 (3H, d, J 7.0 Hz), 2.38 (1H, m), 2.62 (1H, dd, J 13.5, 10.5 Hz), 3.06 (1H, dd, J 13.5, 3.5 Hz), 3.78 (1H, m), 3.92 (1H, dd, J 6.0, 4.5 Hz), 3.96 (1H, dd, J 6.5, 1.0 Hz), 4.01 (1H, dd, J 6.0, 1.5 Hz), 4.11 (1H, s), 5.34 (1H, dd, J 6.5, 4.0 Hz), 6.06 (1H, d, J 9.5 Hz), 7.03–7.30 (6H, m); 13C (125 MHz, CDCl3) 20.7, 37.3, 38.4, 58.4, 64.1, 66.6, 76.2, 88.4, 127.6, 127.7, 129.1, 129.2, 134.9, 151.7, 157.5, 196.0.
Acetamide 8
A solution of cycloadduct 6f (68 mg, 0.19 mmol) in EtOAc–AcOH (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5 mL) was continuously passed through a Pd/C cartridge at 80 °C on the H-Cube hydrogenation setup working in full hydrogenation mode until TLC indicated disappearance of compounds running mid-plate in hexane–EtOAc 2
1, 5 mL) was continuously passed through a Pd/C cartridge at 80 °C on the H-Cube hydrogenation setup working in full hydrogenation mode until TLC indicated disappearance of compounds running mid-plate in hexane–EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture. Solvents were removed under vacuum and to the residual ammonium acetate salt was added DCM (10 mL), Et3N (0.5 mL, excess) and acetic anhydride (0.2 mL, excess). The resulting mixture was stirred for 6 h and concentrated. The residue was purified by flash column chromatography (40–60 petrol–EtOAc 3
1 mixture. Solvents were removed under vacuum and to the residual ammonium acetate salt was added DCM (10 mL), Et3N (0.5 mL, excess) and acetic anhydride (0.2 mL, excess). The resulting mixture was stirred for 6 h and concentrated. The residue was purified by flash column chromatography (40–60 petrol–EtOAc 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the product 8 (17.4 mg, 50%) as a colourless oil: [α]20D = +115.5 (c = 1.01, CHCl3); λmax(thin film) 3490 (br m), 2950 (m), 1720 (s), 1650 (s); m/z (ES+) found 184.0981 (MH+) C9H14NO3 requires 184.0974; 1H (500 MHz, CDCl3) δ 1.68 (1H, dd, J 14.0, 1.5 Hz), 2.09 (3H, s), 2.13 (1H, m), 2.28 (1H, dd, J 13.0, 7.5 Hz), 2.41 (1H, dd, J 17.0, 8.0 Hz), 2.58–2.76 (2H, m), 4.20 (1H, d, J 8.0 Hz), 4.44 (1H, app t, J 6.5 Hz), 4.52 (1H, ddd, J 13.0, 10.0, 6.5 Hz). 13C (125 MHz, CDCl3) 3.35, 6.7, 15.1, 20.4, 53.8, 58.8, 63.8, 157.2, 197.9.
1) to give the product 8 (17.4 mg, 50%) as a colourless oil: [α]20D = +115.5 (c = 1.01, CHCl3); λmax(thin film) 3490 (br m), 2950 (m), 1720 (s), 1650 (s); m/z (ES+) found 184.0981 (MH+) C9H14NO3 requires 184.0974; 1H (500 MHz, CDCl3) δ 1.68 (1H, dd, J 14.0, 1.5 Hz), 2.09 (3H, s), 2.13 (1H, m), 2.28 (1H, dd, J 13.0, 7.5 Hz), 2.41 (1H, dd, J 17.0, 8.0 Hz), 2.58–2.76 (2H, m), 4.20 (1H, d, J 8.0 Hz), 4.44 (1H, app t, J 6.5 Hz), 4.52 (1H, ddd, J 13.0, 10.0, 6.5 Hz). 13C (125 MHz, CDCl3) 3.35, 6.7, 15.1, 20.4, 53.8, 58.8, 63.8, 157.2, 197.9.
Acknowledgements
We would like to thank DEL NI for studentships (CS and PM), Almac for a studentship (YMB), Dr P Nockemann (QUB) for the X-ray analysis and Dr G Trevitt (Almac) for the use of the H-Cube.Notes and references
- Original report of high temperature conditions: J. B. Hendrickson and J. S. Farina, J. Org. Chem., 1980, 45, 3359 CrossRef CAS.
- Base promoted reactions: J. B. Hendrickson and J. S. Farina, J. Org. Chem., 1980, 45, 3361 CrossRef CAS; P. G. Sammes and L. J. Street, J. Chem. Soc., Perkin Trans. 1, 1983, 1261 RSC.
- For a recent review and examples see: V. Singh, U. M. Krishna, V. Vikrant and G. K. Trivedi, Tetrahedron, 2008, 64, 3405 CrossRef CAS.
- R. Ratnayake, D. Covell, T. T. Ranson, K. R. Gustafson and J. A. Beutler, Org. Lett., 2009, 11, 57 CrossRef CAS.
- K. C. Nicolaou, Q. Kang, S. Y. Ng and D. Y.-K. Chen, J. Am. Chem. Soc., 2010, 132, 8219 CrossRef CAS.
- Y. Zhang, T. Wang, Y. Pei, H. Hua and B. Feng, J. Antibiot., 2002, 55, 693 CrossRef CAS.
- K. Tchabanenko, P. McIntyre and J. F. Malone, Tetrahedron Lett., 2010, 51, 86 CrossRef CAS.
- H. Xiong, R. P. Hsung, L. Shen and J. M. Hahn, Tetrahedron Lett., 2002, 43, 4449 CrossRef CAS; Z. Song, T. Lu, R. P. Hsung, Z. F. Al-Rashid, C. Ko and Y. Tang, Angew. Chem., Int. Ed., 2007, 46, 4069 CrossRef; C. Ko, R. P. Hsung, Z. F. Al-Rashid, J. B. Feltenberger, T. Lu, Y. Wei, J. Yang and C. A. Zificsak, Org. Lett., 2007, 9, 4459 CrossRef; T. Lu, Z. Song and R. P. Hsung, Org. Lett., 2008, 10, 541 CrossRef.
- O. Achmatowicz jun., P. Bukowski, B. Szechner, Z. Zwierzchowska and A. Zamojski, Tetrahedron, 1971, 27, 1973 CrossRef.
- B. B. Snider and J. F. Grabowski, Tetrahedron, 2006, 62, 5171 CrossRef CAS.
- M. Bosch and M. Schlaf, J. Org. Chem., 2003, 68, 5225 CrossRef CAS.
- T. B. Nguyen, A. Martel, R. Dhal and G. Dujardin, J. Org. Chem., 2008, 73, 2621 CrossRef CAS.
- Calculations showing endo preference for oxidopyrylium cycloadditions: S. C. Wang and D. J. Tantillo, J. Org. Chem., 2008, 73, 1516 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C spectra for cycloadducts 6a–i and acetamide 8. CCDC reference number 856583. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ob07007c |
| This journal is © The Royal Society of Chemistry 2012 |


