In situ mechanical analysis of cardiomyocytes at nano scales
Yuansheng
Liu†
ab,
Jiantao
Feng†
b,
Liang
Shi
c,
Ruibin
Niu
d,
Quanmei
Sun
b,
Hao
Liu
e,
Jing
Li
b,
Jihong
Guo
a,
Jihong
Zhu
a and
Dong
Han
*b
aEmergency Department, Peking University People's Hospital, Beijing, 100044, People's Republic of China
bNational Center for Nanoscience and Technology, Beijing, 100190, People's Republic of China. E-mail: dhan@nanoctr.cn; Fax: +86 010 62656765; Tel: +86 010 82545568
cDepartment of Stomatoiogy, 306 Hospital, Beijing, 100101, People's Republic of China
dClinical Laboratory, Ordos Central Hospital, Ordos, 017000, People's Republic of China
eIntensive Care Unit, the First People's Hospital of Hangzhou City, Hangzhou, 310006, People's Republic of China
First published on 7th November 2011
Abstract
Nanomechanical behaviors of single living cardiomyocytes are quantitatively observed using calculated torsions and deflections of an AFM cantilever. The lateral contractions are related to the calcium intensity within rather than the vertical beating power of the cardiomyocytes. Drug-induced nanomechanical changes of cardiomyocytes were further investigated by measuring lateral contractions in real time.
Freed from connective tissue and endothelium, isolated cardiomyocytes have been used as an important preparation for studying cardiac mechanics.1 With every heartbeat, cardiomyocytes are subjected to substantial mechanical stretch which also maintains cardiac function. Abnormal mechanical stretch may trigger cardiac remodelling because of cardiomyocyte apoptosis or cardiac fibroblasts proliferation.2 It was reported that patients with diabetic cardiomyopathy or hypertensive heart disease displayed an abnormal cardiac contractile function.3,4 Thus, measuring contractions of cardiomyocytes is necessary for researching the mechanism of heart diseases. Contraction force was usually obtained by measuring globally cell shortening from the displacement of cell extremities. Various transducer designs have been introduced for mechanical measurements, including commercial capacitive force transducers, optical fibers, carbon fibers, thin steel foils, suction pipettes, glass needles, microfabricated polysilicon beams and so on.5,6 However, the spatio–temporal variation of the distribution of strain fields in the cardiomyocyte during the progress of contraction,7 indicates the importance of detecting contraction force at subcellular level. Due to its nanoscale cantilever tips, atomic force microscope (AFM) is capable of measuring contraction force at nano scales. Here, we employed AFM combined with confocal scanning laser microscope, named bio-AFM, to study the mechanical characteristics and related molecular mechanism of cardiomyocytes and their mechanical reactions to ibutilide, a classical drug used to treat arrhythmia.
Utilizing a weak force sensitive silicon cantilever, AFM can be employed to detect mechanical properties of myocytes. Three AFM studies have probed dynamic subcellular mechanics of in vitro cultured myocytes, one investigated the mechanical pulsing of single cardiomyocyte as a function of the lateral position on the cells,8 and one showed AFM tip resonance behavior qualitatively reflecting the periodicity of cell stiffness,9 and another presents quantitative measurement of transverse spatiotemporal stiffness.10In vitro cultured myocytes almost adhere to the substrate, resulting in their main contractions being in the vertical direction. All three studies used vertical deflection of the AFM cantilever to calculate the beating power or stiffness of myocytes. As the physiological state of acutely isolated cardiomyocytes is similar to their in vivo condition, they contract mainly in a lateral way. Such a contraction mainly leads to torsion of the AFM cantilever. Hence, the torsional force of the cantilever, which can be calculated using lateral torsion, may be a more appropriate parameter to characterize mechanics of the acutely isolated cardiomyocytes. However, no research has been reported yet about calculating contraction force of myocytes using lateral torsion of the AFM cantilever. Therefore, we monitored both deflection and torsion of the AFM cantilever dynamically to demonstrate the mechanical character of acutely isolated adult rat ventricular myocytes, while observing the calcium waves with a confocal microscope simultaneously. Because the curvature radius of the cantilever tip is no more than 100 nm, we regard the measured contraction force as a mico–nano scale mechanical feature of the cardiomyocytes.
Myofibrils organize in parallel over the surface of acutely isolated rat ventricular myocytes (Fig. 1(a)). The formation of a myofibril involves the precise ordering of multiple subunits into a linear array of sarcomeres,11 which were arranged mainly by actin and myosin filaments.12 Such an arrangement form an ordered micro/nanocomposite structure which functions as a unit of contraction. Cardiomyocyte contraction occurs when thin actin filaments and thick myosin filaments slide past each other periodically, which is driven by the hydrolysis of adenosine triphosphate (ATP).13 In order to detect the contractions of cardiomyocytes, an AFM silicon cantilever with a conical tip was held on the surface of cardiomyocyte perpendicular to the orientation of the myofibrils (Fig. 1(b)), which was also the direction of the contractions. In Fig. 1(b), the rectangular zone shows the position of the tip. Contractions of a cardiomyocyte can be demonstrated by the torsion of cantilever and torsional force (Ft), calculated according to the formula
| Ft = (θ Gbh3) / 3L(d + h/2) |
| Fn = (ΔV)KS |
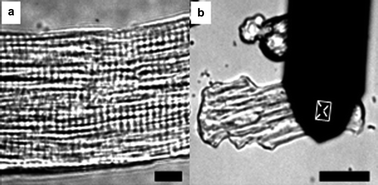 | ||
| Fig. 1 (a) A typical arrangement of sarcomeres over the surface of cardiomyocytes. Bar = 10 μm. (b) AFM cantilever and its position on the surface of a cardiomyocyte. Bar = 50 μm. | ||
The AFM (Agilent Co.) was operated with a silicon cantilever (Budget Sensors) with a typical force constant of 0.223 N m−1. Nominal curvature radius of the tip was no more than 10 nm. Change of the intracellular Ca2+ concentration was assessed via a confocal scanning laser microscope (Yokogawa) fitted onto an inverted microscope (Nikon TE2000U). Such an AFM–Confocal combined imaging system, which we named bio-AFM, enabled us to realize detecting the contraction force and Ca2+ waves of the cardiomyocytes simultaneously.
Adult rat ventricular myocytes were isolated as follows, using procedures that have been reported before.15 The animals’ care was in accordance with institutional guidelines. The protocol in this manuscript was agreed in advance by Ethics Committees of National Center for Nanoscience and Technology, China. In brief, the heart of the adult male Wistar rat was removed quickly under chloral hydrate anesthesia. Perfusion was performed with Ca2+-free Krebs–Hepse solution for three minutes followed by enzyme solution containing collagenase (type II) for twenty minutes. Ventricular tissue was cut into small pieces and digested with collagenase (type II). Digested tissue was then centrifuged followed by suspending cardiomyocytes with bovine serum albumin solution three times. Finally, separated cardiomyocytes were recalcified and anchored in the petri dish containing laminin solution. To observe the changes in calcium intensity, cardiomyocytes were incubated in Krebs–Hepse solution containing 5 mM Fluo4-AM (Biotium) for 30 min and imaged in Krebs–Hepse solution containing 1 mM Ca2+.
The cantilever of the AFM vertically forced to the surface of cardiomyocytes with a loading force about 11 nN. To acquire the contractions of the cardiomyocytes, the feedback loop to keep the cantilever constant was turned off when the cantilever arrived at the setpoint of deflection. Raw vertical movement and torsion of the AFM cantilever were recorded for 142 s and real-time changes of the two kinds of signal were detected. Contractions of a cardiomyocyte was 20.7–49.1 nN and 2.6–11.8 nN calculated with the raw deflection signal and torsion signal, respectively. However, the torsion signal was more sensitive and reliable than the deflection signal (Fig. 2); as Fig. 2 shows, from 46.4 s to 48.4 s, a weak contraction force (arrow) of about 2.6 nN which was detected with the torsion signal (Friction) and no positive signal was recorded in the deflection; moreover, from 60.4 s to 62 s, the vertical deflection signal missed a larger force (arrow) of about 10 nN according to the torsion signal. It indicated that real-space shortening of the sarcomere within cardiomyocyte mainly triggers contraction of the cell, leading to lateral deformation over the cell surface. Thus, the torsion signal of the AFM cantilever may more accurately describe the mechanical function of cardiomyocytes.
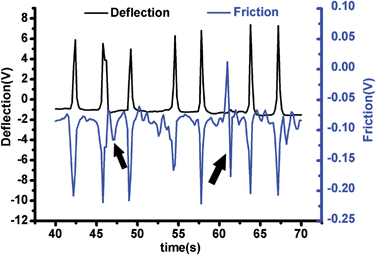 | ||
| Fig. 2 Simultaneous detection of torsional force (Ft) and vertical loading force (Fn) of the AFM cantilever pressed on a cardiomyocte. | ||
Fluorescence signal was collected with the AFM signal simultaneously (Fig. 3). During the cardiac action potential, Ca2+ enters the cell through depolarization-activated Ca2+ channels. Ca2+ entry triggers Ca2+ release from the sarcoplasmic reticulum (SR) through ryanodine receptors (RyR), which is called Ca2+-induced Ca2+-release (CICR) mechanism.16 When the SR Ca2+ content was above a threshold level, in a wave shape, waves of Ca2+ release propagated away from the sites of initial release resulting in a large Ca2+ transient.17Fig. 3(a) shows the fluorescent image of a myocyte and the area (red circle) which the AFM cantilever contacted with. Fig. 3(b) and Fig. 3(c) show calcium intensity and contraction force as a function of time; F/F0 represents the Ca2+ level of the whole cell and F0 is the resting Fluo-4 fluorescence. The Ca2+ waves of the observed myocyte propagated the length of the cell at a speed of 21–48 μm s−1. Duration of each peak in Fig. 3(b) represents the propagation time of a Ca2+ wave in one cycle and it turned out to be 2.6–5.9 s. Duration of each peak in Fig. 3(c) represents the length of time from depolarization to repolarization of the area where the AFM cantilever contacted (Fig. 3(a)). And the de- and repolarization time was 0.6–1.6 s. Frequency of the contraction and the Ca2+ wave was almost the same, with 40 and 38 peaks in 142 s. Scatter charts of calcium intensity with Ft and Fn were plotted, respectively (Fig. 3 (d) and (e)); a strong correlation between calcium intensity and Ft was shown by performing linear regression analysis (r = 0.59, p = 0.0002), indicating calcium–contraction coupling; however, such a correlation was not significant between calcium intensity and Fn. Thus, torsional force is more related to calcium intensity of cardiomyocytes than the vertical loading force is.
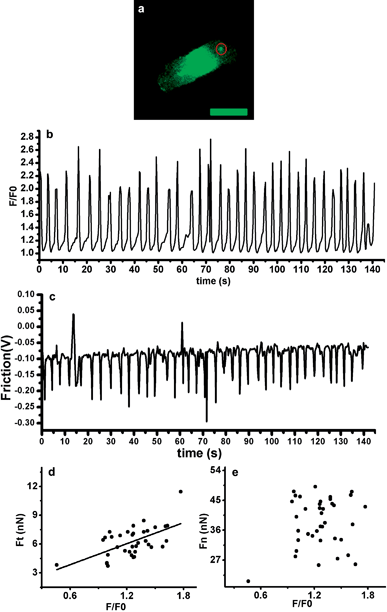 | ||
| Fig. 3 (a) Ca2+ fluorescent image of the cardiomyocyte. Bar = 50 μm. (b) Calcium intensity of the cell measured as a function of time. (c) Torsional force of the cell measured as a function of time. (d) Scatter chart plotted between calcium intensity and Ft; (e) Scatter chart plotted between calcium intensity and Fn. | ||
Using torsional force of the cantilever which was calculated from the lateral deflection, we could test the mechanical changes of the myocytes when exposed to clinical medicine. As a methanesulfonamide derivative with structural similarities to the antiarrhythmic agent sotalol, ibutilide has often been applied to terminate the atrial fibrillation and atrial flutter.18 In a canine model of recent myocardial infarction, ibutilide was also effective at suppressing inducible ventricular arrhythmias,19 the mechanical mechanism of which in single cardiomyocyte level has not been reported yet. In our experiment, before isolated adult rat ventricular myocytes were exposed to 10μM ibutilide, the contraction force was monitored with AFM for 100 s; then, 70 s later, another 100 s was taken to detect the contraction force. Fig. 4(a) shows the contraction force (as a function of time) before and after dosing; the time when the ibutilide was added is shown by the arrow. Contraction frequency was increased from 0.59 Hz to 0.83 Hz by ibutilide addition; we also analyzed the influence of ibutilide on contractions of cardiomyocytes and found a marked decrease by performing Wilcoxon Two-Sample Test (p < 0.0001) (Fig. 4(b)). We assume that ibutilide can prevent ventricular arrhythmias by suppressing the contraction force of the cardiomyocytes.
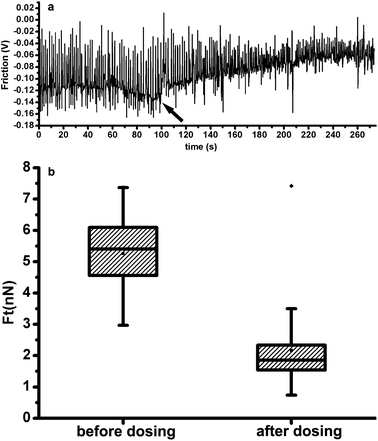 | ||
| Fig. 4 (a) Contraction of a cardiomyocyte, before and after the addition of ibutilide, as a function of time. (b) Box chart showing the distributions of contraction force before and after the addition of ibutilide. | ||
In conclusion, this is the first time to characterize the nanomechanical properties of acutely isolated cardiomyocytes with the torsional signal of an AFM cantilever. And torsion signal was proven to be more accurate than deflection signal to calculate contractions of cardiomyocytes by AFM. Utilizing our home-made AFM–confocal combined imaging system, we can also detect the change of signal molecular fluorescence simultaneously. By monitoring the contraction force reaction of cardiomyocytes to the addition of ibutilide, we speculated that ibutilide can prevent ventricular arrhythmias by suppressing the contraction force of the cardiomyocytes. Consequently, this technology not only offers a possibility to research the differences of mechanical characteristics and related molecular mechanisms between normal cells and dysfunctional cells, but also would possibly open a door on screening drugs which have a potential effect for curing heart diseases by analyzing mechanical changes of cardiomyocytes.
Acknowledgements
This work was supported by the National Science Foundation of China (Grant No. 90709054), the project of 973 in Ministry of Science and Technology of China (Grant Nos. 2006CB705600 and 2009CB930200), and research funding projects of Peking University People's Hospital.References
- G. Iribe, M. Helmes and P. Kohl, Am. J. Physiol.: Heart Circ. Physiol., 2007, 292, H1487 CrossRef CAS.
- L. Xu Dong, W. Xiao Hui, J. Hai Jing, C. Lan Ying and C. Quan, Cell Res., 2004, 14, 16–26 CrossRef.
- S. Boudina and E. D. Abel, Rev. Endocr. Metab. Disord., 2010, 11, 31–39 CrossRef.
- B. A. Borlaug, C. S. P. Lam, V. L. Roger, R. J. Rodeheffer and M. M. Redfield, J. Am. Coll. Cardiol., 2009, 54, 410–418 CrossRef.
- C. Tasche, E. Meyhöfer and B. Brenner, Am. J. Physiol.: Heart Circ. Physiol., 1999, 277, H2400 CAS.
- S. Nishimura, S. Yasuda, M. Katoh, K. P. Yamada, H. Yamashita, Y. Saeki, K. Sunagawa, R. Nagai, T. Hisada and S. Sugiura, Am. J. Physiol.: Heart Circ. Physiol., 2004, 287, H196 CrossRef CAS.
- A. Kamgoué, J. Ohayon, Y. Usson, L. Riou and P. Tracqui, Cytometry A, 2009, 75, 298–308 CrossRef.
- J. Domke, W. J. Parak, M. George, H. E. Gaub and M. Radmacher, Eur. Biophys. J., 1999, 28, 179–186 CrossRef CAS.
- S. G. Shroff, D. R. Saner and R. Lal, Am J Physiol, 1995, 269, C286 CAS.
- E. U. Azeloglu and K. D. Costa, Am. J. Physiol.: Heart Circ. Physiol., 2010, 298, H853 CrossRef CAS.
- G. A. Dabiri, K. K. Turnacioglu, J. M. Sanger and J. W. Sanger, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 9493 CrossRef CAS.
- A. Skwarek-Maruszewska, P. Hotulainen, P. K. Mattila and P. Lappalainen, J. Cell Sci., 2009, 122, 2119 CrossRef CAS.
- I. Rayment, H. M. Holden, M. Whittaker, C. B. Yohn, M. Lorenz, K. C. Holmes and R. A. Milligan, Science, 1993, 261, 58 CAS.
- W. Ma, Y. Sun, D. Han, W. Chu, D. Lin and D. Chen, Microsc. Res. Tech., 2006, 69, 784–793 CrossRef.
- H. Eid, D. M. Larson, J. P. Springhorn, M. A. Attawia, R. C. Nayak, T. W. Smith and R. A. Kelly, Circ. Res., 1992, 71, 40–50 CAS.
- D. M. Bers, Nature, 2002, 415, 198–205 CrossRef CAS.
- Y. Li, M. E. Díaz, D. A. Eisner and S. O'Neill, J. Physiol., 2009, 587, 1283–1292 CAS.
- K. T. Murray, Circulation, 1998, 97, 493 CAS.
- L. V. Buchanan, G. Kabell, U. M. Turcotte, M. N. Brunden and J. K. Gibson, J. Cardiovasc. Pharmacol., 1992, 19, 256–263 CrossRef CAS.
Footnote |
| † These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2012 |
