Highly efficient polymer solar cells based on poly(carbazole-alt-thiophene-benzofurazan)
Bin
Zhang
a,
Xiaowen
Hu
a,
Minquan
Wang
b,
Huiping
Xiao
a,
Xiong
Gong
ac,
Wei
Yang
*a and
Yong
Cao
a
aState Key Laboratory of Luminescent Materials and Devices, Institute of Polymer Optoelectronic Materials and Devices, South China University of Technology, Guangzhou 510640, China. E-mail: pswyang@scut.edu.cn; Fax: +86-020-87110606
bSchool of Material Science & Engineering, South China University of Technology, Guangzhou 510640, China
cCollege of Polymer Science and Polymer Engineering, The University of Akron, 250 South Forge Street, Akron, Ohio 44325-0301, USA
First published on 9th July 2012
Abstract
An octyloxy substituted benzofurazan based poly(carbazole-alt-thiophene-benzofurazan) (PCzDTBF) was synthesized by Suzuki polycondensation. The absorption spectra of the polymer were peaked at 385 and 540 nm, and the HOMO and LUMO energy levels were −5.34 and −3.46 eV, respectively. The hole mobility was found to be 4.8 × 10−4 cm2 V−1 S−1 by the space charge limited current (SCLC) method, and the surface energy (Es) was calculated to be 35.8 mJ m−2 from the contact-angle measurement. Polymer solar cells (PSCs) based on the blend of PCzDTBF and PCBM with a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were fabricated. The devices were found to have a power conversion efficiency (PCE) of 4.2% in the device configuration of ITO/PSS:PEDOT/PCzDTBF:PC70BM/Al. By utilizing poly[(9,9-dioctyl-2,7-fluorene)-alt-(9,9-bis(3′-(N,N-dimethylamino)propyl)-2,7-fluorene)] (PFN) as the cathode interfacial layer, the PCE was enhanced to 5.48%, with an open-circuit voltage (Voc) of 0.90 V, a short-circuit current density (Jsc) of 7.73 mA cm−2, and a fill factor (FF) of 67%. The high efficiencies could be ascribed to the good morphology, resulting from the matched surface energies of the PCzDTBF and PCBM components, and the distinct enhancement on Voc and FF by the PFN layer, where it is possible that a built-in field potential exists between the active layer and cathode.
2 were fabricated. The devices were found to have a power conversion efficiency (PCE) of 4.2% in the device configuration of ITO/PSS:PEDOT/PCzDTBF:PC70BM/Al. By utilizing poly[(9,9-dioctyl-2,7-fluorene)-alt-(9,9-bis(3′-(N,N-dimethylamino)propyl)-2,7-fluorene)] (PFN) as the cathode interfacial layer, the PCE was enhanced to 5.48%, with an open-circuit voltage (Voc) of 0.90 V, a short-circuit current density (Jsc) of 7.73 mA cm−2, and a fill factor (FF) of 67%. The high efficiencies could be ascribed to the good morphology, resulting from the matched surface energies of the PCzDTBF and PCBM components, and the distinct enhancement on Voc and FF by the PFN layer, where it is possible that a built-in field potential exists between the active layer and cathode.
Introduction
Polymer solar cells (PSCs) are becoming increasingly more and more attractive as renewable energy resources because of their versatile merits, such as low cost, flexibility, light weight, and large-scale fabrication.1,2 Some PSCs with a high power conversion efficiency (PCE), overwhelming 8%, were achieved, which were potentially applicable in commercial power generation.3Bulk-heterojunction (BHJ) is one kind of the widely utilized device structure, based on a blend of electron-donating materials (donor) and electron-accepting moieties (acceptor). So far, fullerene derivatives have been the best electron acceptor candidates, such as [6,6]-phenyl-C61-butyric acid methyl ester (PC60BM) and [6,6]-phenyl-C71-butyric acid methyl ester (PC70BM). Therefore, many studies have been focused on the development of electron donors, for instance, polythiophenes (PThs),4–7 polybenzodithiophenes (PBDTs),8,9 polyfluorenes (PFs),10 and polysilafluorenes (PSiFs),11 In addition, polycarbazoles (PCzs) were star polymers for PSCs, because of the fantastic characteristics of carbazoles, such as high hole mobility, deep highest occupied molecular orbit (HOMO) energy levels, high thermal stability and feasible molecular structure tuning.12,13 In the donor polymers, the mostly used molecular architecture was donor–acceptor–donor (D–A–D), in which the donor was an electron-rich unit (such as thiophene and furan), and the acceptor refers to the electron-deficient unit (such as, benzothiadiazole (BT),12,13 benzoselenodizole14 and quinoxaline15). Benzofurazan (BF),16–19 contains a higher electronegative oxygen, compared with the sulfur atom in the BT unit, which can induce the deeper HOMO energy level. However, the solubility of BT and BF based D–A–D monomers is poor in organic solvents (e.g., toluene) due to their high polarity and planarity. Therefore, the side chain substituted BT20 and BF21–23 units can improve the solubility tremendously in common solvents, and make it more feasible to synthesize the polymers with high molecular weights and good solubility.
Previously, the alcohol soluble poly[(9,9-dioctyl-2,7-fluorene)-alt-(9,9-bis(3′-(N,N-dimethylamino)propyl)-2,7-fluorene)] (PFN) was utilized as the cathode interfacial layer in photovoltaic devices, where the photovoltaic performances were improved, possibly because of the existence of built-in electric fields between the active layer and the cathode.24,25 Therefore, the insertion of an alcohol soluble PFN layer as the cathode interfacial layer is an attractive strategy to achieve highly efficient PSCs.
Here, an alternating conjugated polymer containing an octyloxy substituted BF unit and 9-octyl-2,7-carbazole was synthesized, and used as a donor material in BHJ solar cells. Moreover, the PFN was used as the cathode interfacial layer, which ultimately contributed to the large enhancement in the photovoltaic efficiency because of the possible distinct improvements in FF and Voc.
Experimental section
Characterization and instrumentation
Both 1H nuclear magnetic resonance (NMR) and 13C NMR measurements were carried out on a Bruker 300 MHz DRX spectrometer with tetramethylsilane (TMS) as the internal reference. Mass spectrometry was performed on a Bruker Esquire HCT PLUS. The number-average molecular weight (Mn) and weight-average molecular weight (Mw) were determined on a Waters gel permeation chromatography (GPC) system with linear polystyrene as the standard and chloroform containing 0.25 v/v% triethylamine as eluent. Cyclic voltammetry (CV) was characterized on a CHI600D electrochemical workstation with a standard three electrode cell based on a platinum (Pt) working electrode and a Pt wire counter electrode, against a saturated calomel electrode (SCE) as the reference electrode, at a scan rate of 50 mV s−1 within a nitrogen-saturated anhydrous solution of 0.1 mol L−1 tetrabutylammonium hexafluorophosphates (Bu4NPF6) in acetonitrile, versus ferrocene/ferrocenium (Fc/Fc+) as the internal reference. UV-vis absorption spectra were performed on a HP 8453 spectrophotometer. Tapping-mode atomic force microscopy (AFM) was carried out for the identification of topography and blend film phase images on a Veeco Nanoscope V scanning probe microscope. Surface energy was measured on Dataphysics OCA40 Micro by calculations from the surface tension data of diiodomethane and water.Device fabrication
All devices were fabricated on ITO-coated glass substrates. A thin-layer (40 nm) of PEDOT:PSS (Clevios 4083) was spin-coated onto UV-ozone treated ITO substrates at 4000 rpm for 40 s and then baked at 140 °C for 15 min in air. The solution containing the polymer (10 mg ml−1) and fullerene (20 mg ml−1) (polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fullerene = 1
fullerene = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, wt/wt) in o-dichlorobenzene (ODCB) were spin-coated inside a glove-box with nitrogen at 1200 rpm for 40 s to form the active layers (∼80 nm). For the device structure of ITO/PEDOT-PSS/active layer/PFN/Al, the PFN layer (5 nm) was prepared by spin-casting a mixed solution (0.2 mg ml−1) with a methanol to acetic acid volume ratio of 100
2, wt/wt) in o-dichlorobenzene (ODCB) were spin-coated inside a glove-box with nitrogen at 1200 rpm for 40 s to form the active layers (∼80 nm). For the device structure of ITO/PEDOT-PSS/active layer/PFN/Al, the PFN layer (5 nm) was prepared by spin-casting a mixed solution (0.2 mg ml−1) with a methanol to acetic acid volume ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 before the cathode evaporation. The aluminum cathode (80 nm) was then thermally evaporated under vacuum (∼10−6 Torr) through a shadow mask defining an active device area of 0.16 cm2. The current–voltage (J–V) curves were measured using a Keithley 2400 multimeter under AM 1.5 G solar illumination at 87 mW cm−2 and a Thermal-Oriel 150 W solar simulator. Incident photon-to-current conversion efficiency (IPCE) values were obtained with a monochromator and calibrated with a silicon photodiode. Hole mobility data of neat polymer and PCzDTBF:PCBM blend films were measured by the space charge limited current (SCLC) method with the device configuration of ITO/PEDOT:PSS (40 nm)/active layer (80–100 nm)/MoO3 (10 nm)/Al (90 nm). Preliminarily, PEDOT:PSS was spin casted on the ITO coated glass substrate, then, active layers were prepared from the ODCB solution with a 1% concentration by spin coating. Finally, MoO3 and Al layers were deposited by thermal evaporation under high vacuum, respectively.
1 before the cathode evaporation. The aluminum cathode (80 nm) was then thermally evaporated under vacuum (∼10−6 Torr) through a shadow mask defining an active device area of 0.16 cm2. The current–voltage (J–V) curves were measured using a Keithley 2400 multimeter under AM 1.5 G solar illumination at 87 mW cm−2 and a Thermal-Oriel 150 W solar simulator. Incident photon-to-current conversion efficiency (IPCE) values were obtained with a monochromator and calibrated with a silicon photodiode. Hole mobility data of neat polymer and PCzDTBF:PCBM blend films were measured by the space charge limited current (SCLC) method with the device configuration of ITO/PEDOT:PSS (40 nm)/active layer (80–100 nm)/MoO3 (10 nm)/Al (90 nm). Preliminarily, PEDOT:PSS was spin casted on the ITO coated glass substrate, then, active layers were prepared from the ODCB solution with a 1% concentration by spin coating. Finally, MoO3 and Al layers were deposited by thermal evaporation under high vacuum, respectively.
Synthesis of monomers and polymer
The synthetic routes were displayed in Scheme 1. 1,2-Bis(octyloxy) benzene,26 1,2-dinitro-4,5-bis(octyloxy)benzene and 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxa borolan-2-yl)-9-octyl-carbazole20 were synthesized according to the published procedures. All the other monomers and the polymer were obtained by the following synthetic methodologies. | ||
| Scheme 1 Synthetic routes of monomers and polymer. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM = 10
DCM = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as mixed eluent, and yielded 2.0 g of the target compound as a yellow solid. 1H NMR (300 MHz, CDCl3, δ): 8.46 (dd, J = 3.84 Hz, 2H, ArH), 7.50 (dd, J = 5.16 Hz, 2H, ArH), 7.22 (t, 2H, ArH), 4.15 (t, 4H, CH2), 1.98 (m, 4H, CH2), 1.47–1.30 (br, 20H, CH2), 0.90 (t, 6H, CH3). 13C NMR (75 MHz, CDCl3, δ): 151.73, 146.77, 132.92, 130.85, 127.98, 127.12, 113.06, 74.51, 31.79, 30.26, 29.48, 29.23, 25.87, 22.63, 14.05. MS (APCI): Calcd, 540.8; found (M + 1)+, 541.8.
1 as mixed eluent, and yielded 2.0 g of the target compound as a yellow solid. 1H NMR (300 MHz, CDCl3, δ): 8.46 (dd, J = 3.84 Hz, 2H, ArH), 7.50 (dd, J = 5.16 Hz, 2H, ArH), 7.22 (t, 2H, ArH), 4.15 (t, 4H, CH2), 1.98 (m, 4H, CH2), 1.47–1.30 (br, 20H, CH2), 0.90 (t, 6H, CH3). 13C NMR (75 MHz, CDCl3, δ): 151.73, 146.77, 132.92, 130.85, 127.98, 127.12, 113.06, 74.51, 31.79, 30.26, 29.48, 29.23, 25.87, 22.63, 14.05. MS (APCI): Calcd, 540.8; found (M + 1)+, 541.8.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, PDI = 2.1.
500, PDI = 2.1.
Results and discussion
Synthesis
Detailed synthetic routes of the monomers and polymer are shown in Scheme 1. The polymer PCzDTBF was synthesized by Suzuki polycondesation in toluene, and purified by Soxhlet extraction in methanol, acetone, hexane and chloroform with 100 ml for 12 h each, and was then precipitated in methanol. The solubility of the polymer is good in organic solvents (such as tetrahydrofuran, chloroform, chlorobenzene, dichlorobenzene), because of the octyloxy substituted on the BF unit. The gel permeation chromatography (GPC) characterization shows that the number-average molecular weight (Mn) and weight-average molecular weight (Mw) are 6000 and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, respectively, with a polydispersity index (PDI) of 2.1 (Table 1). The PCzDTBF does not show a higher molecular weight, which is possibly because the octyl side chain in the carbazole unit makes the polymer solubility limited and ultimately inhibits the increment of the molecular weight during polymerization.19
500, respectively, with a polydispersity index (PDI) of 2.1 (Table 1). The PCzDTBF does not show a higher molecular weight, which is possibly because the octyl side chain in the carbazole unit makes the polymer solubility limited and ultimately inhibits the increment of the molecular weight during polymerization.19
Absorption and electrochemical analysis
The UV-vis absorption spectra of PCzDTBF in solution and in film are presented in Fig. 1a. The absorption spectra peaks at 385 and 540 nm are attributed to the localized π–π* transition and the internal charge transfer (ICT) interaction between the donor and acceptor units, respectively.22 Compared with the absorption in solution, the absorption spectrum in film displays peaks that are a little broadened, which implicates the amorphous film and no further molecular stacking.22 The onset of the absorption spectrum in film is 644 nm, which corresponds to the optical band gap of 1.93 eV (Table 1).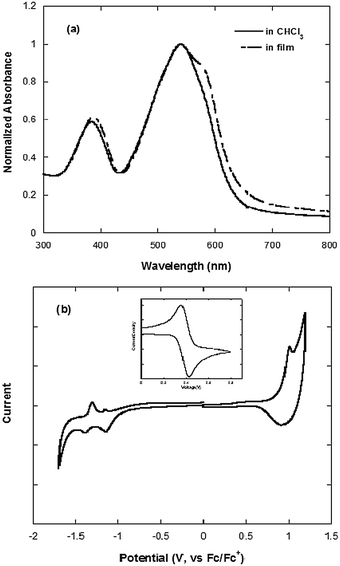 | ||
| Fig. 1 UV-vis absorption spectra of PCzDTBF in chloroform and in film (a); cyclic voltammogram of PCzDTBF in film (b) (inset: graph of the ferrocene oxidative curve). | ||
The cyclic voltammogram (CV) characterization (Fig. 1b) presents the reversibly oxidative and reductive curves, in which the onsets of the oxidation and reduction potentials are located at 0.93 and −0.95 V versus SCE, respectively. The redox potential of the Fc/Fc+ internal reference is 0.39 V vs. SCE. The highest occupied molecular orbit (HOMO) and the lowest unoccupied molecular orbit (LUMO) energy levels, determined by calculating the empirical formula of EHOMO = −e(Eox + 4.8 − E1/2, (Fc/Fc+)) and ELUMO = −e(Ere + 4.8 − E1/2, (Fc/Fc+)), are −5.34 and −3.46 eV, respectively.5 The energy band gap of PCzDTBF from the CV data is 1.88 eV, which is approximate to the optical band gap of 1.93 eV (Table 1).
Photovoltaic properties
PSCs were fabricated with the configurations of ITO/PEDOT:PSS/PCzDTBF:PCBM/Al and ITO/PEDOT:PSS/ PCzDTBF:PCBM/PFN/Al, respectively. The current density–voltage (J–V) curves are shown in Fig. 2a. We can see that the short circuit current density (Jsc) and the open circuit voltage (Voc) of PC70BM based device are larger than the ones of PC60BM as acceptor. Furthermore, the insertion of PFN as the cathode interfacial layer made the Voc and fill factor (FF) enhanced distinctly, and therefore, resulted in the power conversion efficiency (PCE) increased ultimately. In Fig. 2b, the incident photon-to-current conversion efficiency (IPCE) of PC70BM based devices are broadened and exhibits better photo-to-current conversion in the visible region with approximate 10% improvement compared with the PC60BM device, which is possibly ascribed to the intensified and broadened absorption of the PCzDTBF:PC70BM based blend film. | ||
| Fig. 2 Current density-voltage (J–V) characteristics of PCzDTBF: PCBM under AM 1.5 G, 87 mW cm−2 (a); incident photon-to-current conversion efficiency (IPCE) and UV-vis absorption spectra of PCzDTBF:PCBM blend films (b). | ||
Table 2 shows the distinctive increment in Jsc values and PCEs from 6.61 mA cm−2 and 3.63% for PC60BM to 7.36 mA cm−2 and 4.20% for the PC70BM device, respectively. Compared with the devices without a PFN layer, the performances with PFN as a cathode interfacial layer were enhanced dramatically. Even though the Jsc increased only a little, the Voc and FF were distinctly enhanced, where the Voc increased with maximal 0.1 V, and FF realized a 10% enhancement. Ultimately, the PCE reached 5.48%, which had more than a 30% increment both in PC60BM and PC70BM based photovoltaic devices.
Active layer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) 2, w/w) |
Cathode | J sc (mA cm−2) | V oc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| PCzDTBF:PC60BM | Al | 6.61 | 0.80 | 60 | 3.63 |
| PFN/Al | 6.67 | 0.90 | 69 | 4.73 | |
| PCzDTBF:PC70BM | Al | 7.36 | 0.85 | 57 | 4.20 |
| PFN/Al | 7.73 | 0.90 | 67 | 5.48 |
Hole mobility and film morphology
The hole mobility, both in neat polymer and blend films, of PCzDTBF:PCBM were measured by the space charge limited current (SCLC) method with the device configuration of ITO/PEDOT:PSS/active layer/MoO3/Al. Fig. 3 shows the J–V curves of the PCzDTBF and blend films from SCLC devices. The hole mobilities (Table 3) were 4.8 × 10−4, 2.5 × 10−4 and 2.1 × 10−4 cm2 V−1 s−1 for PCzDTBF, PCzDTBF:PC60BM and PCzDTBF:PC70BM, respectively, which were comparable to that of the polycarbazole based polymers.16,20 Compared with the neat polymer, the hole mobilities of the blend films decreased by about half, but still sustained the same order of magnitude, which would provide a sufficient hole transporting ability in the photovoltaic devices and pay extensive contribution to the PCE.27 | ||
| Fig. 3 J–V characteristics of PCzDTBF and blend films from SCLC devices. | ||
| Active layer | Thickness (nm) | μ (×10−4 cm2 V−1 s−1) |
|---|---|---|
| PCzDTBF | 100 | 4.8 |
| PCzDTBF:PC60BM | 92 | 2.5 |
| PCzDTBF:PC70BM | 82 | 2.1 |
AFM images of the blend films are shown in Fig. 4. The root-mean-square (RMS) roughness of the PC60BM and PC70BM based films are 0.58 and 0.72 nm, respectively, which shows that both films are very smooth. Moreover, the phase images (Fig. 4c–d) display the nano-scale phase separation,28 which can support the effective holes and electron transporting channels and inhibit the charge carriers recombination, and ultimately affect the photovoltaic properties. On the other hand, the miscibility decides the film morphology in blend systems directly. From Table 1, we can see that the surface energy (Es) of PCzDTBF is 35.8 mJ m−2, which is similar to that of the PC60BM of 34.2 mJ m−2.29 The similar Es in the blend components caused well-mixed films, resulting from optimal phase separation of the PCBM domains. This scenario can also depict the evidences for the formation of nano-scale phase separation between PCzDTBF and PC60BM or PC70BM greatly.
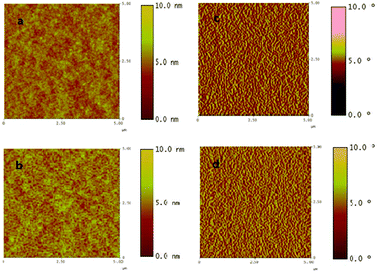 | ||
Fig. 4 AFM images of blend films (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 weight ratio), topography of PCzDTBF:PC60BM (a) and PCzDTBF:PC70BM (b); phase images of PCzDTBF:PC60BM (c) and PCzDTBF:PC70BM (d). 2 weight ratio), topography of PCzDTBF:PC60BM (a) and PCzDTBF:PC70BM (b); phase images of PCzDTBF:PC60BM (c) and PCzDTBF:PC70BM (d). | ||
Compared with the decyloxy substituted BF unit based polycarbazole (PC-DTBX),21PCzDTBF had a shorter octyloxy side chain and higher molecular weight, which may lead to the better device performances. On the other hand, the high FF and nano-scale phase separation could also be effectively contributable to the high photovoltaic properties.
Nevertheless, the weaker absorption of PCzDTBF after 650 nm resulted in the smaller Jsc value compared with other PSCs, in which the Jsc value reached more than 12 mA cm−2.7 Therefore, if the absorption of this polymer can be extended to the longer wavelength, the photovoltaic performances will be escalated to a higher level. Prospective research work is in progress.
Conclusions
An octyloxy substituted BF based polycarbazole (PCzDTBF) was synthesized by Suzuki polycondensation and utilized as a donor material in PSCs. The photovoltaic performance gave a maximal PCE of 4.2% under the device configuration of ITO/PSS:PEDOT/PCzDTBF:PC70BM/Al. By using PFN as the cathode interfacial layer, the PCE was enhanced to 5.48%, with a Voc of 0.90 V, a Jsc of 7.73 mA cm−2, and a FF of 67%. The high efficiencies were possibly attributed to the good nano-scale phase separation between PCzDTBF and PCBM components, resulting from their matchable surface energies, and the distinct improvement of Voc and FF by the PFN layer, where a built-in field potential possibly exists between the active layer and cathode.Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (nos. 50990065, 51010003 and 21074038), and the State Key Basic Research Project of China (nos. 2009CB623600 and 2009CB930604).Notes and references
- Y. F. Li, Acc. Chem. Res., 2012, 45, 723 CrossRef CAS.
- P. M. Beaujuge and J. M. J. Fréchet, J. Am. Chem. Soc., 2011, 133, 20009 CrossRef CAS.
- Z. C. He, C. M. Zhong, X. Huang, W. Y. Wong, H. B. Wu, L. W. Chen, S. J. Su and Y. Cao, Adv. Mater., 2011, 23, 4636 CrossRef CAS.
- Y. Y. Liang, Y. Wu, D. Q. Feng, S. T. Tsai, H. J. Son, G. Li and L. P. Yu, J. Am. Chem. Soc., 2009, 131, 56 CrossRef CAS.
- Y. Y. Liang, D. Q. Feng, Y. Wu, S. T. Tsai, G. Li, C. Ray and L. P. Yu, J. Am. Chem. Soc., 2009, 131, 7792 CrossRef CAS.
- J. H. Hou, H. Y. Chen, S. Q. Zhang, R. I. Chen, Y. Yang, Y. Wu and G. Li, J. Am. Chem. Soc., 2009, 131, 15586 CrossRef CAS.
- H. Y. Chen, J. H. Hou, S. Q. Zhang, Y. Y. Liang, G. W. Yang, Y. Yang, L. P. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649 CrossRef CAS.
- L. J. Huo and J. H. Hou, Polym. Chem., 2011, 2, 2453 RSC.
- J. H. Hou, M. H. Park, S. Q. Zhang, Y. Yao, L. M. Chen, J. H. Li and Y. Yang, Macromolecules, 2008, 41, 6012 CrossRef CAS.
- O. Inganäs, F. L. Zhang, K. Tvingstedt, L. M. Andersson, S. Hellstrom and M. R. Andersson, Adv. Mater., 2010, 22, E100 CrossRef.
- E. G. Wang, L. Wang, L. F. Lan, C. Luo, W. L. Zhuang, J. B. Peng and Y. Cao, Appl. Phys. Lett., 2008, 92, 033307 CrossRef.
- N. Blouin, A. Michaud and M. Leclerc, Adv. Mater., 2007, 19, 2295 CrossRef CAS.
- S. H. Park, A. Roy, S. Beaupré, S. Cho, N. Coates, J. S. Moon, D. Moses, M. Leclerc, K. Lee and A. J. Heeger, Nat. Photonics, 2009, 3, 297 CrossRef CAS.
- W. Zhao, W. Z. Cai, R. X. Xu, W. Yang, X. Gong, H. B. Wu and Y. Cao, Polymer, 2010, 51, 3196 CrossRef CAS.
- E. G. Wang, L. T. Hou, Z. Q. Wang, S. Hellström, F. L. Zhang, O. Inganäs and M. R. Andersson, Adv. Mater., 2010, 22, 5240 CrossRef CAS.
- N. Blouin, A. Michaud, D. Gendron, S. Wakim, E. Blair, R. Neagu-Plesu, M. Belletete, G. Durocher, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2008, 130, 732 CrossRef CAS.
- J. C. Bijleveld, M. Shahid, J. Gilot, M. M. Wienk and R. A. J. Janssen, Adv. Funct. Mater., 2009, 19, 3262 CrossRef CAS.
- C. V. Hoven, X. D. Dang, R. C. Con, J. Peet, T. Q. Nguyen and G. C. Bazan, Adv. Mater., 2010, 22, E63 CrossRef CAS.
- W. Y. Nie, C. M. MacNeill, Y. Li, R. E. Noftle, D. L. Carroll and R. C. Coffin, Macromol. Rapid Commun., 2011, 32, 1163 CrossRef CAS.
- R. P. Qin, W. W. Li, C. H. Li, C. Du, C. Veit, H. F. Schleiermacher, M. Andersson, Z. S. Bo, Z. P. Liu, O. Inganäs, U. Wuerfel and F. L. Zhang, J. Am. Chem. Soc., 2009, 131, 14612 CrossRef CAS.
- P. Ding, C. M. Zhong, Y. P. Zou, C. Y. Pan, H. B. Wu and Y. Cao, J. Phys. Chem. C, 2011, 115, 16211 CAS.
- J. M. Jiang, P. A. Yang, H. C. Chen and K. H. Wei, Chem. Commun., 2011, 47, 8877 RSC.
- J. M. Jiang, P. A. Yang, T. H. Hsieh and K. H. Wei, Macromolecules, 2011, 44, 9155 CrossRef CAS.
- J. Luo, H. B. Wu, C. He, A. Y. Li, W. Yang and Y. Cao, Appl. Phys. Lett., 2009, 95, 043301 CrossRef.
- C. He, C. M. Zhong, H. B. Wu, R. Q. Yang, W. Yang, F. Huang, G. C. Bazan and Y. Cao, J. Mater. Chem., 2010, 20, 2617 RSC.
- J. L. Sessler, W. B. Callaway, S. P. Dudek, R. W. Date and D. W. Bruce, Inorg. Chem., 2004, 43, 6650 CrossRef CAS.
- J. D. Kotlarski, D. J. D. Moet and P. W. M. Blom, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 708 CrossRef CAS.
- G. J. Zhao, Y. J. He and Y. F. Li, Adv. Mater., 2010, 22, 4355 CrossRef CAS.
- J. S. Kim, Y. M. Lee, J. H. Lee, J. H. Park, J. K. Kim and K. Cho, Adv. Mater., 2010, 22, 1355 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2012 |
