Improved biodistribution, pharmacokinetics and photodynamic efficacy using a new photostable sulfonamide bacteriochlorin†
Janusz M.
Dąbrowski
*a,
Luis G.
Arnaut
*bc,
Mariette M.
Pereira
b,
Krystyna
Urbańska
d and
Grażyna
Stochel
a
aFaculty of Chemistry, Jagiellonian University, Ingardena 3, 30-060 Krakow, Poland. E-mail: jdabrows@chemia.uj.edu.pl; Fax: +48126340515; Tel: +48126632293
bChemistry Department, University of Coimbra, Rua Larga, Coimbra, Portugal. E-mail: lgarnaut@ci.uc.pt; Fax: +35123982770
cLuzitin S.A., R. Bayer 16, 3045-016 Coimbra, Portugal
dFaculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, Gronostajowa 7, 30-387 Krakow, Poland
First published on 6th February 2012
Abstract
The bacteriochlorin-mediated PDT effects on melanoma tumors were investigated in correlation with its biodistribution. The pharmacokinetics of the photostable 5,10,15,20-tetrakis(2,6-dichloro-3-N-ethylsulfamoylphenyl)bacteriochlorin was determined in DBA mice bearing S91 melanoma tumors at different time intervals (2 h–72 h) after i.p. injection of a 10 mg kg−1 drug dose. PDT efficacy was maximal when irradiation was performed 24 h after i.p. administration, and led to the complete disappearance of tumors for nearly 2 months. Compared to the analogue sulfonated compound, the median tumor growth delay with respect to the control group increased from 27 to 44 days. This improvement is attributed to the higher stability, higher absorption in the NIR, amphiphilicity, and better selectivity of the sulfonamide bacteriochlorin.
Introduction
Photodynamic therapy (PDT) is based on the generation of reactive oxygen species (ROS) by excitation of an administered photosensitizer with visible or, preferably, NIR radiation.1 The photosensitizer absorbs light and becomes activated from a ground singlet state to a short-lived excited singlet state. Then it returns to the ground state via fluorescence, or more preferably it converts into a triplet state that reacts with substrate molecules by electron transfer with the formation of free radicals (type I reaction), or transfers its energy to ground-state molecular oxygen generating singlet oxygen (type II reaction).2 These highly cytotoxic species can oxidize important biological molecules such as proteins, lipids and nucleic acids inducing cell death, vascular damage and inflammation.3 Much efforts have been directed towards the development of photosensitizers with strong absorption in the NIR,4–7 where tissues are more transparent. We recently showed that the cytotoxicity of singlet oxygen and hydroxyl radicals can be combined in strongly NIR absorbing bacteriochlorins to yield more potent photosensitizers for PDT.6,7Through the use of these new compounds, in combination with improved protocols, the efficiency of the photodynamic effect has been significantly enhanced. Some of the problems not yet adequately solved by clinically approved photosensitizers, such as Photofrin® or Foscan®, are the poor tumor selectivity and the prolonged cutaneous photosensitivity due to their slow elimination from the body.8,9 Although the mechanism of tumor retention of the sensitizer is not yet clear, there is evidence that the amphiphilic character of the photosensitizer is an important factor.10 Several approaches have been proposed in order to modify the lipophilicity of the photosensitizer.10–12 Exchanging sulfonic to sulfonamide groups in the tetrapyrrolic macrocycle results in a significant enhancement of photocytotoxicity in vitro7 due to the increase in lipophilic character of the sensitizing molecule, and it can be expected to improve selectivity towards tumors.
PDT in vivo requires a balance between photostability, accumulation in tumors, low dark toxicity, high photodynamic efficacy and rapid clearance from the body after the treatment. Photostability allows the same photosensitizer molecule to generate large amounts of ROS and does not compromise pharmacokinetics. Accumulation in tumors increases the bioavailability of the photosensitizer and, together with low dark toxicity, increases the therapeutic index. 5,10,15,20-Tetrakis(2,6-dichloro-3-N-ethylsulfamoylphenyl)bacteriochlorin (Cl2BEt) was recently shown to be very photostable, highly up-taken by cancer cells, localized preferentially in the endoplasmic reticulum and exhibiting high phototoxicity in vitro.7 Additionally we expect Cl2BEt to provide a compromise between very hydrophobic sensitizers which have low biocompatibility and precipitate in biological media, and very hydrophilic dyes such as ClBOH or ClCOH, shown in Scheme 1, which are less efficient photosensitizers because they mostly localize in the tumor stroma.13
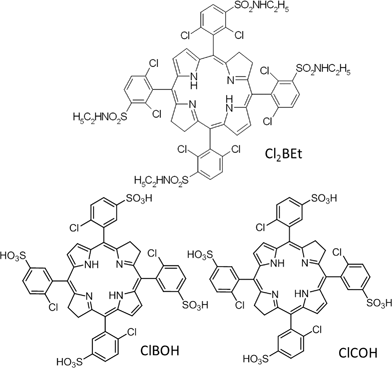 | ||
| Scheme 1 Chemical structure of 5,10,15,20-tetrakis(2,6-dichloro-3-N-ethylsulfamoylphenyl)bacteriochlorin (Cl2BEt), 5,10,15,20-tetrakis(2-chloro-5-sulfophenyl)bacteriochlorin (ClBOH) and 5,10,15,20-tetrakis(2-chloro-5-sulfophenyl)chlorin (ClCOH). | ||
The aim of this first in vivo study with halogenated sulfonamide bacteriochlorins is to provide insight into the pharmacokinetics, biodistribution and photodynamic efficacy of this class of photosensitizers in S91 melanoma bearing mice, and to assess their PDT efficacy with respect to that of halogenated and sulfonated bacteriochlorins.
Results and discussion
Preparation and spectroscopic characterization
The cost-effective, large scale and environmentally friendly method of the efficient synthesis of 5,10,15,20-tetrakis(2,6-dichloro-3-N-ethylsulfamoylphenyl)bacteriochlorin (Cl2BEt, Scheme 1) starting from dichloroporphyrins and amphiphilic sulfamoyl side chain (NHethyl) was described elsewhere14 and all the characterization data are in good agreement with published data.In brief, the halogenated tetraphenylporphyrin was prepared by condensation of pyrrole with the desired 2,6-dichlorobenzaldehyde or 2-chlorobenzaldehyde using acetic acid/nitrobenzene as the solvent.15 Chlorosulfonation of the halogenated tetraphenylporphyrin followed by nucleophilic substitution with ethylamine and water provided the desired amphiphilic sulfonamide halogenated porphyrins which were finally reduced to the corresponding bacteriochlorins using solid p-toluenesulfonylhydrazide, both as the reagent and as the solvent, as the hydrogen source and in the absence of solvents or bases.14
Cl2BEt exhibits the characteristic bacteriochlorin absorption spectrum with a split near-UV Soret feature, a near-infrared Qy band of comparable intensity and only a narrow band in the green (∼510 nm) that is typical for this class of compounds.16
Representative absorption and fluorescence spectra for Cl2BEt are shown in Fig. 1. The Qy(0,0) absorption maximum of bacteriochlorin in ethanol is placed at 746 nm, and the Qy(0,0) fluorescence peak is shifted by ∼2 nm. The absorption coefficient in the infrared, εmax = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1, is among the largest for non-aggregating molecules in polar solvents. The shoulder at 410 nm and the small peak at 658 nm are evidence of a small chlorin contamination, which is present in less than 5% in our samples. With appropriate selection of excitation and emission wavelengths, this contamination does not affect our results.
000 M−1 cm−1, is among the largest for non-aggregating molecules in polar solvents. The shoulder at 410 nm and the small peak at 658 nm are evidence of a small chlorin contamination, which is present in less than 5% in our samples. With appropriate selection of excitation and emission wavelengths, this contamination does not affect our results.
 | ||
| Fig. 1 Electronic absorption and fluorescence spectra of Cl2BEt measured in ethanol at room temperature. | ||
The fluorescence quantum yield of these bacteriochlorins is rather low (ΦF = 0.008) but it is still sufficient to use these photosensitizers as therapeutic agents and diagnostic tools at the same time, which allows monitoring therapy progress. The explanation of the reduced fluorescence is the presence of chlorine atoms, which provide the internal heavy atom effect. These strongly electron-withdrawing groups also contribute to stabilize the bacteriochlorin, avoiding oxidation.
Biodistribution and pharmacokinetic
The biodistribution of Cl2BEt was evaluated in vivo using S91 melanoma bearing DBA2 mice. The use of animals for this experimental study was approved by the Jagiellonian University Committee for Ethics of Experiments on Animals (decisions no. 89/2008 from 11 December 2008 and no. 11/2011 from 23 February 2011). Two hours after the photosensitizer injection, in all organs a minimum level of bacteriochlorin was observed but the highest concentration of the bacteriochlorin was found in the spleen. After 6 hours the maximum concentration of bacteriochlorin was measured in the spleen and liver. This level is higher than in blood at any point of time. The highest concentration of the compound in the blood was recorded at 12 hours, which is certainly related to the mode of administration (intraperitoneal injection). High concentrations of the compound after 6 and 12 hours were also detected in the intestines. This demonstrates its rapid elimination from the body.The amount of photosensitizer in different organs after 24 hours from injection of the compound is as follows: spleen > liver > tumor > lungs > heart > intestine > kidneys > blood > skin > muscle. The results are summarized in Table S2 in the ESI†. Most remarkably, the distribution of the bacteriochlorin to the tumor was much higher than blood, muscle and skin (Fig. 2). However, kidneys showed a significant uptake of the conjugate at 24 h and 48 h, with a considerable decrease at 72 h post-injection, suggesting glomerular filtration of the conjugate by the kidneys. The photosensitizer showed a greater uptake in tumor vs. skin and vs. muscle at 24 h post-injection, indicating some degree of tumor selectivity. The tumor-to-skin ratio of 6 and tumor-to-muscle ratio of 7 are significantly higher than those of the sulfonated bacteriochlorin (ClBOH).6 These ratios are also higher than tumor-to-skin and tumor-to-muscle ratios reported for Photofrin® in mice with implanted HT29 cells at the same time after i.p. administration (1 and 5, respectively).17 The more favourable biodistribution and pharmacokinetics of Cl2BEt than those of ClBOH can be related to the significant differences in their polarity and stability. ClBOH is much less stable than Cl2BEt and circulates in the body for a shorter time. Cl2BEt stays longer in the body and thus has a better chance to accumulate in the tumor. The selectivity may also be explained by the values of the n-octanol–water partition constants (POW), which are log POW = −1.7 for ClBOH and 1.83 for Cl2BEt.14 This is a good balance of lipophilicity because extremely lipophilic compounds may aggregate in the body and their aggregated states may have reduced photodynamic activity. However, very lipophilic drugs may require several weeks or months to clear from the skin. On the other hand, too hydrophilic or unstable photosensitizers may be eliminated from the body within a few minutes without having a chance to accumulate in the tumors.18
![Biodistribution of Cl2BEt expressed as its concentration [μg g−1] in wet tissue in the tumor, in the surrounding skin and muscle and in the blood as a function of the time after i.p. injection at a dose of 10 mg kg−1 to the DBA2 mice with S91 tumors. Each point represents the standard error of mean ± SEM of 3 animals.](/image/article/2012/MD/c2md00308b/c2md00308b-f2.gif) | ||
| Fig. 2 Biodistribution of Cl2BEt expressed as its concentration [μg g−1] in wet tissue in the tumor, in the surrounding skin and muscle and in the blood as a function of the time after i.p. injection at a dose of 10 mg kg−1 to the DBA2 mice with S91 tumors. Each point represents the standard error of mean ± SEM of 3 animals. | ||
Tumor response to bacteriochlorin-PDT
We selected subcutaneously implanted S91 tumors with diameters between 0.5 and 0.7 cm to study the PDT efficacy of Cl2BEt. 24 hours after intraperitoneal injection of Cl2BEt (10 mg kg−1 in formulation ethanol/PEG/PBS, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), the tumors were irradiated with laser (746 nm) at 80–90 mW cm−2. During irradiation mice were anesthetized with inhaled Foran®. After the treatment mice were observed for several weeks.
5), the tumors were irradiated with laser (746 nm) at 80–90 mW cm−2. During irradiation mice were anesthetized with inhaled Foran®. After the treatment mice were observed for several weeks.
Our previous work on the photodynamic efficacy of sulfonated bacteriochlorin (ClBOH) showed that this compound excited with the NIR light results in inhibition of tumor growth after PDT for about 50 days. However, in all cases tumor regrowth was observed before 60 days. An analogous sulfonated chlorin (ClCOH) exhibited a very similar PDT efficacy, with median tumor growth delays of 27 and 29 days, for ClBOH and ClCOH, respectively.19 The modest photostability of ClBOH probably biased its PDT efficacy with respect to the more weakly light-absorbing chlorin, but in both cases excess hydrophilicity may impair the outcome of PDT. Hydrophilic photosensitizers are usually located in the tumor stroma, which may result in a lower photodynamic efficiency.13
A similar experiment was performed for sulfonamide bacteriochlorin (Cl2BEt), which is more hydrophobic and characterized by more favourable spectroscopic and photophysical properties (Fig. 1). After irradiation with a single dose of 96 J cm−2 the complete disappearance of the tumor was observed. Hyperaemia, oedema and the progressive darkening of the tumor were observed. After twelve hours, necrotic scab formation was observed in the tumor and in its immediate surroundings (Fig. 3).
 | ||
| Fig. 3 Pictures of DBA 2 mice bearing S91 melanoma tumors before PDT and PDT-treated animals using Cl2BEt as the photosensitizer and NIR laser light. | ||
Tumor response to PDT performed at 24 hours after i.p. injection of bacteriochlorin is shown in Fig. 4 and 5. Compared with the control group, PDT treatments at different intervals all have significant anti-tumor effects. Initial tumor regression together with pronounced oedema was observed shortly after treatment, and the tumors generally became necrotic and flat within 1–2 days. PDT treatment 24 hours post-i.p. injection also led to some skin damage with oedema and large scab formation.
 | ||
| Fig. 4 Tumor disappearance following PDT treatment with i.p. administration of 10 mg kg−1 sulfonamide bacteriochlorin (Cl2BEt) and irradiation with diode laser (746 nm) 24 h post-injection compared with PDT treatment with sulfonated bacteriochlorin (ClBOH) and control (untreated). | ||
 | ||
| Fig. 5 Kaplan–Meier curve of S91 tumor regrowth after PDT treatment with sulfonamide (Cl2BEt) and sulfonated (ClBOH) 24 h after photosensitizer injection. The tumor-bearing mice were irradiated with 96 J cm−2 or 108 J cm−2 dose of light (80 and 90 mW respectively, 746 nm) at 24 h post-i.p. injection of 10 mg kg−1 drug dose. | ||
Treatments with sulfonamide bacteriochlorin or light alone exhibited no antitumor effects. Eventually all tumors reappeared as the mice were followed for 80 days. Nevertheless, the median tumor growth delay was 44 days, and an increase by a factor of 1.6 with respect to the sulfonated bacteriochlorin as it is compared in the Kaplan Meyer curve is presented above.
Conclusions
This work focused on the photodynamic activity of a sulfonamide bacteriochlorin in vivo against poorly pigmented melanoma S91 growing under the skin of DBA2 mice. The determination of the time depended accumulation and retention of a photosensitizer in the tumor and healthy surrounding tissue was taken into consideration in the optimization of the PDT protocol. Biodistribution studies established that the sulfonamide bacteriochlorin appears in all tested mouse tissues and organs after intraperitoneal injection. Determination of the tumor to normal tissue ratios allowed the selection of optimal time for the irradiation of the tumor. The largest amount of the photosensitizer in the tumor was observed 24 hours after its i.p. administration. Interestingly, this sulfonamide bacteriochlorin has a very favourable retention time, because 96 h after administration its level in the analyzed tissues was very low. This sulfonamide bacteriochlorin is a much more effective photosensitizer than its sulfonated analogues. This improvement can be rationalized by photochemical properties already described in the literature.20,21 Cl2BEt combines very favourable spectroscopic properties, such as high absorption in the NIR, with a long triplet lifetime and high quantum yields of ROS (both singlet oxygen and hydroxyl radical), high photostability and amphiphilicity. Additionally, Cl2BEt also has favourable pharmacodynamics, namely selective localization in the tumor tissue and rapid clearance after treatment, which make it a very efficient photosensitizer for PDT using NIR.Acknowledgements
This work is a part of the ERA Chemistry Project supported by The National Centre for Research and Development (PL, grant number 60303), and Foundation for Science and Technology (PT, grant number 0002/2008). JMD thanks the Ministry of Science and Higher Education for Iuventus Plus grant no. IP2011009471. Some research equipment was financed by the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (contract no. POIG.02.01.00-12-023/08).Notes and references
- D. Dolmans, E. D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS.
- R. W. Redmond, in Advances in Photodynamic Therapy: Basic, Translational, and Clinical, ed. M. R. Hamblin and P. Mroz, Artech House, Boston and London, 2008, pp. 41–58 Search PubMed.
- A. P. Castano, P. Mroz and M. R. Hamblin, Nat. Rev. Cancer, 2006, 6, 535–545 CrossRef CAS.
- B. G. Ongarora, X. Hu, H. Li, F. R. Fronczek and M. G. H. Vicente, Med. Chem. Commun., 2012, 3, 179–194 RSC.
- M. Garcıa-Diaz, D. Sanchez-Garcia, J. Soriano, M. L. Sagrista, M. Mora, A. Villanueva, J. C. Stockert, M. Canete and S. Nonel, Med. Chem. Commun., 2011, 2, 616–619 RSC.
- J. M. Dabrowski, K. Urbanska, L. G. Arnaut, M. M. Pereira, A. R. Abreu, S. Simões and G. Stochel, ChemMedChem, 2011, 6, 465–475 CrossRef CAS.
- J. M. Dabrowski, L. G. Arnaut, M. M. Pereira, K. Urbanska, S. Simões, G. Stochel and L. Cortes, Free Radical Biol. Med., 2012, 52, 1188-1200 CrossRef.
- D. A. Bellnier, W. R. Greco, G. M. Loewen, H. Nava, A. R. Oseroff and T. J. Dougherty, Lasers Surg. Med., 2006, 38, 439–444 CrossRef.
- T. J. Dougherty, M. T. Cooper and T. S. Mang, Lasers Surg. Med., 1990, 10, 485–488 CrossRef CAS.
- K. R. Adams, M. C. Berembaum, R. Bonnett, A. N. Nizhnik and A. Salgado, J. Chem. Soc., Perkin Trans. 1, 1992, 1465–1470 RSC.
- R. Bonnett, A. N. Nizhnik, R. D. White and M. C. Berembaum, J. Photochem. Photobiol., B, 1990, 6, 29–37 CrossRef CAS.
- G. Zheng, W. R. Potter, S. H. Camacho, J. R. Missert, G. Wang, D. A. Bellnier, B. W. Henderson, M. A. J. Rodgers, T. J. Dougherty and R. K. Pandey, J. Med. Chem., 2001, 44, 1540–1559 CrossRef CAS.
- R. W. Boyle and D. Dolphin, Photochem. Photobiol., 1996, 64, 469–485 CrossRef CAS.
- M. M. Pereira, C. J. P. Monteiro, A. V. C. Simoes, S. M. A. Pinto, A. R. Abreu, G. F. F. Sa, E. F. F. Silva, L. B. Rocha, J. M. Dabrowski, S. J. Formosinho, S. Simoes and L. G. Arnaut, Tetrahedron, 2010, 66, 9545–9551 CrossRef CAS.
- J. M. Dabrowski, M. M. Pereira, L. G. Arnaut, C. J. P. Monteiro, A. F. Peixoto, A. Karocki, K. Urbanska and G. Stochel, Photochem. Photobiol., 2007, 83, 897–903 CrossRef CAS.
- L. G. Arnaut, Adv. Inorg. Chem., 2011, 63, 187–234 CrossRef CAS.
- A. Hajri, S. Wack, C. Meyer, M. K. Smith, C. Leberquier, M. Kedinger and M. Aprahamian, Photochem. Photobiol., 2002, 75, 140–148 CrossRef CAS.
- O. Mazor, A. Brandis, V. Plaks, E. Neumark, V. Rosenbach-Belkin, Y. Salomon and A. Scherz, Photochem. Photobiol., 2005, 81, 342–351 CrossRef CAS.
- J. M. Dabrowski, M. Krzykawska, K. Urbanska, L. G. Arnaut, M. M. Pereira, C. Monteiro, S. Simões and G. Stochel, ChemMedChem, 2011, 6, 1715–1726 CrossRef CAS.
- E. F. F. Silva, C. Serpa, J. M. Dabrowski, C. J. P. Monteiro, S. J. Formosinho, G. Stochel, K. Urbaska, S. Simões, M. M. Pereira and L. G. Arnaut, Chem.–Eur. J., 2010, 16, 9273–9286 CrossRef CAS.
- J. M. Dabrowski, L. G. Arnaut, M. M. Pereira, C. J. P. Monteiro, K. Urbanska, S. Simoes and G. Stochel, ChemMedChem, 2010, 5, 1770–1780 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, spectroscopic and photophysical properties, concentration of the photosensitizer in different organs. See DOI: 10.1039/c2md00308b |
| This journal is © The Royal Society of Chemistry 2012 |
