A second generation MRI contrast agent for imaging zinc ions in vivo†
Luis M.
De León-Rodríguez
*a,
Angelo J. M.
Lubag
b,
Jorge A.
López
a,
Gabriel
Andreu-de-Riquer
a,
José C.
Alvarado-Monzón
a and
A. Dean
Sherry
b
aDepartamento de Química, Universidad de Guanajuato, Cerro de la Venada s/n, Guanajuato, Gto. C.P. 36040, México. E-mail: lmdeleon@quijote.ugto.mx; Fax: +52 4737326252
bAdvanced Imaging Research Center, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, Texas 75390-9185, USA
First published on 7th March 2012
Abstract
A Zn2+ specific GdDOTA derivative containing two bis-(3-pyrazolyl) units was prepared and characterized. Unlike a previously reported Zn2+ binding agent, the new agent binds to human albumin both in the presence and absence of Zn2+.
Magnetic resonance imaging (MRI) contrast agents (CAs) are widely used in clinical practice to measure dynamic processes or to highlight tissue abnormalities. Most current Gd3+-based, T1 shortening CAs available to clinicians are considered nonspecific, extracellular agents. One exception is the recently approved albumin binding agent, Ablavar,1 an agent that remains largely confined to the vascular blood pool and hence useful for vascular imaging. Other second generation CAs will likely also display high specificity for biomolecular targets or be responsive to specific biological stimuli (e.g. oxidation, metals, enzymes, etc.).2 CAs responsive to zinc ions (Zn2+) are particularly interesting since imbalances in this ion are related to several pathologies including Alzheimer's disease, prostate cancer and diabetes. Brain, prostate and pancreas contain concentrations of Zn2+ high enough to make this ion suitable for imaging by MRI in these organs.3–5 Several Zn2+-responsive CAs have been reported6–10 but few have been shown to work in vivo.10,11 Among the factors that can limit in vivo applications of such sensor for imaging Zn2+ include the binding affinity between the CA and Zn2+ (KD) and the proton longitudinal relaxivity (r1) change that occurs upon binding of Zn2+ ions to the CA. It has been proposed that KD values for any Zn2+ MRI specific CA should be in the ∼μM range for in vivo applications (for single molecule CAs where r1 changes upon stimuli recognition are limited).10 One recently reported MRI Zn2+ specific CA that shows promise for imaging beta cell function in vivo, GdDOTA-diBPEN, forms a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex with Zn2+ (one Zn2+ per BPEN) with a KD = 33.6 nM. A particular feature of this agent is that it shows a modest increase (∼20%) in r1 upon binding to Zn2+ in the absence of albumin (37 °C, pH 7.6, 0.1 M Tris buffer, 23 MHz), but a substantial change in r1 (∼165% from 6.6 to 17.4 mM−1 s−1) upon formation of a 1
2 complex with Zn2+ (one Zn2+ per BPEN) with a KD = 33.6 nM. A particular feature of this agent is that it shows a modest increase (∼20%) in r1 upon binding to Zn2+ in the absence of albumin (37 °C, pH 7.6, 0.1 M Tris buffer, 23 MHz), but a substantial change in r1 (∼165% from 6.6 to 17.4 mM−1 s−1) upon formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex with Zn2+ ions in the presence of fatty acid free human serum albumin (HSA). This enhanced r1 results from binding of GdDOTA-diBPEN(Zn)2 to site 2 of the protein with a KD ≈ 45 μM.10 The r1 enhancement was more modest (∼40%, from 6.1 to 8.6 mM−1 s−1) when the agent was mixed with human blood plasma, likely reflecting more competition with other serum components for binding to HSA. In an effort to maximize the binding interactions between Zn2+ agents like GdDOTA-diBPEN with HSA in serum, a simple modification of the Zn2+ binding arms was first considered. Given the relatively high affinity of GdDOTA-diBPEN(Zn)2 for site 2 of HSA, a largely hydrophobic binding pocket, it is reasonable to assume that the pyridine groups of ZnBPEN adopt a conformation that favors π–π interactions within the site 2 binding cavity.10 Any factor that weakens this interaction in vivo will have a deleterious effect on the observed r1. The BPEN moiety and a number of variations have been explored in the design of Zn2+ sensors.12 Here, we describe the synthesis and characterization of a 3-pyrazolyl version of GdDOTA-diBPEN and explore its utility as a Zn2+ sensor in vivo. The 3-pyrazolyl moiety was chosen because it retains a pyridine-like nitrogen atom (N2) to act as a donor atom for Zn2+ but also has a neighboring N1H group known to stabilize metal ion complexes via added hydrogen bonding interactions.13 This feature could provide additional stability between the Zn2+-agent and HSA.
2 complex with Zn2+ ions in the presence of fatty acid free human serum albumin (HSA). This enhanced r1 results from binding of GdDOTA-diBPEN(Zn)2 to site 2 of the protein with a KD ≈ 45 μM.10 The r1 enhancement was more modest (∼40%, from 6.1 to 8.6 mM−1 s−1) when the agent was mixed with human blood plasma, likely reflecting more competition with other serum components for binding to HSA. In an effort to maximize the binding interactions between Zn2+ agents like GdDOTA-diBPEN with HSA in serum, a simple modification of the Zn2+ binding arms was first considered. Given the relatively high affinity of GdDOTA-diBPEN(Zn)2 for site 2 of HSA, a largely hydrophobic binding pocket, it is reasonable to assume that the pyridine groups of ZnBPEN adopt a conformation that favors π–π interactions within the site 2 binding cavity.10 Any factor that weakens this interaction in vivo will have a deleterious effect on the observed r1. The BPEN moiety and a number of variations have been explored in the design of Zn2+ sensors.12 Here, we describe the synthesis and characterization of a 3-pyrazolyl version of GdDOTA-diBPEN and explore its utility as a Zn2+ sensor in vivo. The 3-pyrazolyl moiety was chosen because it retains a pyridine-like nitrogen atom (N2) to act as a donor atom for Zn2+ but also has a neighboring N1H group known to stabilize metal ion complexes via added hydrogen bonding interactions.13 This feature could provide additional stability between the Zn2+-agent and HSA.
A GdDOTA derivative containing two N,N-bis-(3-pyrazolyl-methyl) ethylene diamine (BPYREN) units (GdDOTA-diBPYREN, Fig. 1) was prepared (see Scheme S1, ESI†). The r1 relaxivity of GdDOTA-diBPYREN increased upon addition of Zn2+ and Cu2+ but did not change with added Ca2+ or Mg2+. The relaxivity of the complex was 4.2 ± 0.1 mM−1 s−1 (37 °C, pH 7.6, 0.1 M Tris buffer, 23 MHz) in the absence of Zn2+ and this gradually increased to 6.9 ± 0.2 mM−1 s−1 with addition of Zn2+ until 2 equiv. had been added, remaining constant with 3 equiv. of Zn2+ (Fig. 2). This is consistent with formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Gd
2 (Gd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn) complex. Mass peaks from GdDOTA-diBPYREN(Zn), adducts of this complex with Na+ and K+, and GdDOTA-diBPYREN(Zn)2 were observed by MALDI-TOF MS spectroscopy confirming the formation of 1
Zn) complex. Mass peaks from GdDOTA-diBPYREN(Zn), adducts of this complex with Na+ and K+, and GdDOTA-diBPYREN(Zn)2 were observed by MALDI-TOF MS spectroscopy confirming the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Gd
2 (Gd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn) complex (Fig. S6 and S7, ESI†). The r1 changes upon metal binding were more dramatic for Zn2+ than for Cu2+.
Zn) complex (Fig. S6 and S7, ESI†). The r1 changes upon metal binding were more dramatic for Zn2+ than for Cu2+.
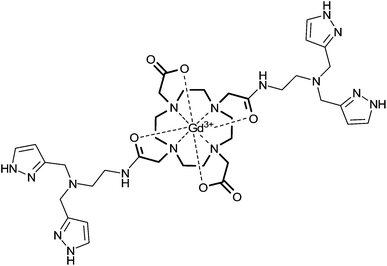 | ||
| Fig. 1 GdDOTA-diBPYREN. For the 3-pyrazolyl units the most stable isomer is shown. | ||
 | ||
| Fig. 2 Relaxivity of GdDOTA-diBPYREN at 23 MHz and 37 °C in the presence of MCl2, where M = Zn2+, Cu2+, Ca2+, or Mg2+. All solutions were prepared in 100 mM Tris buffer at pH 7.6. r1 was determined from the slope of the line of the reciprocal of T1versus the concentration of gadolinium (GdDOTA-diBPYREN was varied from 1 to 5 mM). | ||
Relaxivity changes in the presence of HSA were also measured for GdDOTA-diBPYREN. Here, r1 increased from 8.4 ± 0.2 mM−1 s−1 in the absence of Zn2+ (37 °C, pH 7.6, 0.1 M Tris buffer, 0.6 mM HSA, 23 MHz) to 15.3 ± 0.4 mM−1 s−1 with addition of 3 equiv. of Zn2+. The higher r1 of GdDOTA-diBPYREN in HSA in the absence of Zn2+ compared to GdDOTA-diBPEN (6.6 ± 0.1 mM−1 s−1) suggests that the former might be binding to the protein as well. The relaxivity also increased in a similar magnitude to that of Zn2+ in the presence of Cu2+ in HSA buffered solution but remained unchanged with Ca2+ and Mg2+. For reference the r1 of Prohance™ (GdDO3A), a clinically used CA, was determined to be 2.9 ± 0.1 mM−1 s−1 (37 °C, pH 7.6, 0.1 M Tris buffer with 0.6 mM HSA, 23 MHz) in the absence of Zn2+ and 2.7 ± 0.1 mM−1 s−1 with 3 equiv. of Zn2+.
An observation made during the T1 measurements in the absence of HSA was the slight precipitation of Zn2+ for solutions containing CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn2+ ratios above 1
Zn2+ ratios above 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, indicating a weak binding of Zn2+, which will precipitate at pH 7.6 in the absence of a chelating ligand.
1, indicating a weak binding of Zn2+, which will precipitate at pH 7.6 in the absence of a chelating ligand.
The KD of GdDOTA-diBPYREN with Zn2+ was determined by a competitive assay using the commercially available fluorophore FluoZin-1 (see ESI†). This dye was chosen since it shows a moderate metal binding. Using the experimentally determined dissociation constant for the dye (24.3 ± 2.8 μM), a binding constant of 378.6 ± 83.1 μM was estimated for formation of GdDOTA-diBPYREN(Zn)2 (per BPYREN binding unit). As expected a much weaker affinity for Zn2+ than GdDOTA-diBPEN was found.
Relaxivity measurements were performed in the presence of warfarin or dansylsarcosine, which are known to bind HSA at site 1 or 2 respectively. A 18% decrease in r1 was observed when dansylsarcosine (5 equiv. relative to GdDOTA-diBPYREN) was added to a solution containing agent/Zn2+ 1/2 in HSA buffered solution, while no change in r1 was observed upon addition of warfarin under the same conditions. Also a 16% decrease in r1 was determined when dansylsarcosine was added to GdDOTA-diBPYREN in the absence of Zn2+ while no change was seen when warfarin was added. These results indicate that both GdDOTA-diBPYREN and GdDOTA-diBPYREN(Zn)2 bind to site 2 of subdomain IIIA of HSA.
To gain further insight into binding of the agent to HSA, a change in water proton relaxation rates (ΔR1) was measured under conditions where the concentration of HSA was varied while the concentration of GdDOTA-diBPYREN and Zn2+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) or GdDOTA-diBPYREN alone was maintained constant (Fig. 3). For GdDOTA-diBPYREN alone a KD of 10.7 ± 0.9 μM with HSA was determined (see ESI†). The titration curve of GdDOTA-diBPYREN in the presence of 1
2) or GdDOTA-diBPYREN alone was maintained constant (Fig. 3). For GdDOTA-diBPYREN alone a KD of 10.7 ± 0.9 μM with HSA was determined (see ESI†). The titration curve of GdDOTA-diBPYREN in the presence of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Zn2+ showed quite a different binding feature. At low concentrations of HSA, ΔR1 increased in parallel to that observed for Zn2+ free GdDOTA-diBPYREN but at higher concentrations of HSA ΔR1 decreased. To explain this observation one should consider that HSA has a Zn2+ binding site with a KD of 29.5 nM14 so that the observed behaviour in the titration curve of GdDOTA-diBPYREN with Zn2+ shows that HSA is competing for Zn2+ with the agent as expected given the weaker binding of BPYREN to Zn2+ than HSA.
2 Zn2+ showed quite a different binding feature. At low concentrations of HSA, ΔR1 increased in parallel to that observed for Zn2+ free GdDOTA-diBPYREN but at higher concentrations of HSA ΔR1 decreased. To explain this observation one should consider that HSA has a Zn2+ binding site with a KD of 29.5 nM14 so that the observed behaviour in the titration curve of GdDOTA-diBPYREN with Zn2+ shows that HSA is competing for Zn2+ with the agent as expected given the weaker binding of BPYREN to Zn2+ than HSA.
 | ||
| Fig. 3 Titration of GdDOTA-diBPYREN 1 mM (squares), and GdDOTA-diBPYREN 1 mM plus 2 mM of Zn2+ (circles) with HSA. All measurements were made at 23 MHz and 37 °C in 100 mM Tris buffer at pH 7.6. The solid curves represent the best fit to eqn S(4) in the ESI†. | ||
Fitting the titration curve for HSA < 0.2 M allows one to estimate a KD of 29.4 ± 2.2 μM GdDOTA-diBPYREN(Zn)2 to HSA, which indicates that the agent reported herein has a stronger binding to HSA than GdDOTA-diBPEN(Zn)2 (KD ≈ 45 μM).10
To determine the effectiveness of GdDOTA-diBPYREN as a relaxation agent under physiological conditions, relaxivity measurements were also performed in human blood serum. In this case, r1 increased from 6.0 ± 0.1 to 13.1 ± 0.1 mM−1 s−1 (37 °C, 23 MHz) upon addition of Zn2+ a 114% change which is ∼3 fold larger than that found for GdDOTA-diBPEN. This suggests that the 3-pyrazolyl groups provide added stability between the Zn2+-bound agent and the protein.
Given the relatively large change in r1 observed for GdDOTA-diBPYREN in serum, MR images (9.4T) of 24 h fasted mice were collected before and after glucose stimulated insulin/Zn2+ release from pancreas as reported for GdDOTA-diBPEN.15 Fasting glucose levels were measured in representative animals prior to imaging (∼5.6 ± 0.3 mM, considered normal). After collection of anatomical multi-slice images to locate the pancreas, each mouse was given a bolus injection of glucose standard intraperitoneally (2 mg kg−1 body weight, i.p.) or a saline solution for control mice followed ∼10 min later by a bolus of GdDOTA-diBPYREN or Prohance™ (2.5 μmol; 4 times higher than the amount used of GdDOTA-diBPEN) via a tail vein catheter. With this protocol, T1 weighted images showed a significant enhancement within 10 min after GdDOTA-diBPYREN injection for the animals given a prior bolus of glucose (10 min prior to contrast agent injection) (Fig. 4) which is in agreement to what was previously observed with GdDOTA-diBPEN. This is evidence that GdDOTA-diBPYREN is sensing secretion of Zn2+ from β-cells of the pancreas in response to a glucose stimulus. On the other hand, when no glucose was injected (saline injection) the pancreas region showed a quick and significant contrast enhancement in the pancreas area within 5 min after GdDOTA-diBPYREN injection followed by an immediate decrease (Fig. 5 curve with rhombic symbols). This later behaviour corresponds to what is observed with non-specific CAs (Fig. 5 curve with triangle symbols). The relaxivity change in the presence of HSA for GdDOTA-diBPYREN at 9.4T (400 MHz) was substantially smaller than the increase observed at 23 MHz, as expected for a complex undergoing slow rotation (r1 increased from 3.2 ± 0.2 mM−1 s−1 in the absence of Zn2+ (37 °C, pH 7.6, 0.1 M Tris buffer, 0.6 mM HSA, 400 MHz) to 4.0 ± 0.3 mM−1 s−1 with addition of 2 equiv. of Zn2+). However, this change is still significant for the detection of glucose stimulated Zn2+ secretion in vivo.
 | ||
| Fig. 4 T1-Weighted MR images of the mouse abdomen showing the axial view of the pancreas (duodenal side). Pre-GdDOTA-biPYREN (A), and 10 min post i.v. injection of the CA (B). Images were collected using a FSEMS sequence with the following parameters: TR = 89.03 ms; effective echo time (TE) = 11.21 ms; FOV 30 × 30 mm2, data matrix = 256 × 256, averaging = 3, slice = 1 mm, number of slices = 6, gap = 0; ETL = 1, kzero = 1. No gating was employed. Each image includes phantoms in capillary tubes positioned horizontally and aligned with the mouse body. These phantoms contain GdDOTA-diBPYREN, 25 μM; H2O; GdCl3, 25 μM, Prohance™; 25 μM clockwise from bottom-left to bottom-right. The yellow arrow points the pancreas location. | ||
 | ||
| Fig. 5 Image normalized intensity (n.i.) plot of the duodenal pancreas region. Top graph ■ corresponds to GdDOTA-diBPYREN after i.p. injection of glucose and ◆ corresponds to GdDOTA-diBPYREN after i.p. injection of saline. Bottom graph ▲ corresponds to Prohance™ after i.p. injection of glucose. Image intensity was normalized relative to the highest intensity point for each curve. Agent i.v. injection corresponds to time = 0. | ||
To summarize this work, we report here a new Zn2+ MRI agent containing 3-pyrazolyl groups that has a suboptimal binding affinity for Zn2+ but has an improved binding interaction with HSA. This agent shows the highest reported r1 change in human serum upon Zn2+ recognition. These results suggest that future designs of Zn2+ specific MRI CAs based on the HSA binding principle should consider optimizing interactions between the agent and the protein.
Acknowledgements
This research was supported in part by Universidad de Guanajuato (DAIP) Grant 000024/10 and SEP Grant UGTO-CA-107 and by grants from the National Institutes of Health, USA (DK-058398 and RR-02584) and Robert A. Welch Foundation Grant AT-584.Notes and references
- M. Goyen, Vasc. Health Risk Manage., 2008, 4, 1 CrossRef CAS.
- L. M. De Leon-Rodriguez, A. J. M. Lubag, C. R. Malloy, G. V. Martinez, R. J. Gillies and A. D. Sherry, Acc. Chem. Res., 2009, 42, 948 CrossRef CAS.
- K. Jomova, D. Vondrakova, M. Lawson and M. Valko, Mol. Cell. Biochem., 2010, 345, 91 CrossRef CAS.
- J. Gumulec, M. Masarik, S. Krizkova, V. Adam, J. Hubalek, J. Hrabeta, T. Eckschlager, M. Stiborova and R. Kizek, Curr. Med. Chem., 2011, 18, 5041 CrossRef CAS.
- J. Jansen, W. Karges and L. Rink, J. Nutr. Biochem., 2009, 20, 399 CrossRef CAS.
- K. Hanaoka, K. Kikuchi, Y. Urano and T. Nagano, J. Chem. Soc., Perkin Trans. 2, 2001, 1840 RSC.
- K. Hanaoka, K. Kikuchi, Y. Urano, M. Narazaki, T. Yokawa, S. Sakamoto, K. Yamaguchi and T. Nagano, Chem. Biol., 2002, 9, 1027 CrossRef CAS.
- X. A. Zhang, K. S. Lovejoy, A. Jasanoff and S. J. Lippard, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 10780 CrossRef CAS.
- J. L. Major, G. Parigi, C. Luchinat and T. J. Meade, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 13881 CrossRef CAS.
- A. C. Esqueda, J. A. López, G. Andreu-de-Riquer, J. C. Alvarado-Monzón, J. Ratnakar, A. J. M. Lubag, A. D. Sherry and L. M. De León-Rodríguez, J. Am. Chem. Soc., 2009, 131, 11387 CrossRef CAS.
- T. Lee, X.a. Zhang, S. Dhar, H. Faas, S. J. Lippard and A. Jasanoff, Chem. Biol., 2010, 17, 665 CrossRef CAS.
- E. M. Nolan and S. J. Lippard, Acc. Chem. Res., 2009, 42, 193 CrossRef CAS.
- J. Pérez and L. Riera, Eur. J. Inorg. Chem., 2009, 4913 CrossRef.
- A. J. Stewart, C. A. Blindauer, S. Berezenko, D. Sleep and P. J. Sadler, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 3701 CrossRef CAS.
- A. J. M. Lubag, L. M. De Leon-Rodriguez, S. C. Burgess and A. D. Sherry, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 18400 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental conditions, synthesis, relaxometric and fluorescence experiments and MRI details. See DOI: 10.1039/c2md00301e |
| This journal is © The Royal Society of Chemistry 2012 |
