Potent inhibition of Ca2+-dependent activation of calpain-1 by novel mercaptoacrylates†
Sarah E.
Adams
a,
Christian
Parr
b,
David J.
Miller
a,
Rudolf K.
Allemann
*a and
Maurice B.
Hallett
*b
aSchool of Chemistry, Cardiff University, Main Building, Park Place, Cardiff, CF10 3AT, United Kingdom. E-mail: allemannrk@cardiff.ac.uk; Fax: +44 (0) 29 208 74030; Tel: +44 (0)29 208 79014
bNeutrophil Signalling Group, Institute of Molecular and Experimental Medicine, School of Medicine, Cardiff University, WHRI, Heath Park, Cardiff, CF14 4XN, United Kingdom. Fax: +44 (0)2920 75 2643; Tel: +44 (0)29 2074 2748
First published on 30th November 2011
Abstract
Calpain-1 is a Ca2+-activated cytosolic cysteine protease. Its activation has been linked to extravasation into inflamed tissue of white blood cells, especially neutrophils. Calpain-1 may therefore be an anti-inflammatory target for therapeutic intervention. 24 novel monohalogenated phenyl and indole mercaptoacrylic acid derivatives were synthesised. The location and nature of the ring-coupled halides strongly influenced the potency of these compounds to inhibit calpain activation. Several of the calpain-1 inhibitors showed IC50 values in the low nanomolar range and prevented cell shape change of neutrophils, a necessary prelude to their migration from the blood in the body.
Introduction
Calpains form a family of 14 cytosolic Ca2+-activated cysteine proteases.1 Calpain-1 and calpain-2 are the most widely expressed in the body and are structurally similar;2 each possesses a large subunit of molecular mass 80k, which shares 60% sequence similarity and a common small subunit with a mass of 28k (identical in both forms).3 The small subunit is a regulatory subunit containing a Ca2+-binding region with several helix-loop-helix motifs commonly found in Ca2+ binding proteins (EF-hands) and a glycine rich region.4 The large catalytic subunit also contains a calcium binding domain alongside the catalytic triad. In the absence of Ca2+, residues of the triad are held apart in an inactive form; Ca2+-binding to both subunits induces conformational changes that lead to enzymatic activation.5Although the two enzymes are structurally similar, they are distinguished by the concentration of Ca2+ required to activate each of them. Calpain-1 requires micromolar (10–50 μM) and calpain-2 millimolar (0.2–0.35 mM) concentrations of Ca2+ for activation. This difference in Ca2+-sensitivity gives rise to the alternative nomenclature for the enzymes of μ- and m-calpain.6
Although both enzymes have been implicated in regulating aspects of cell behaviour,7 calpain-1 is especially important in controlling cell shape changes that occur during neutrophil migration8 and platelet aggregation.9 In addition, activation of calpains has been linked to diseases with inflammatory components such as rheumatoid arthritis, Alzheimer's disease and ischemic strokes.1,10 Thus calpains represent potential drug targets, inhibition of which may provide therapeutic benefit.
Small molecule and peptide-based inhibitors of calpain activity have been reported in the literature. The peptidic inhibitors target the proteolytic site and show little selectivity, inhibiting a range of cysteine proteases in addition to calpains.11 In contrast, two mercaptoacrylic acid derivatives act as potent inhibitors through the Ca2+-regulatory site and hence are calpain specific (Fig. 1).12 They show selectivity for inhibition of calpain-1 over calpain-2;12 PD150606 (1) and PD151746 (2) inhibit calpain-1 with Ki values of 0.21 μM and 0.26 μM and calpain-2 with Ki values of 0.37 μM and 5.33 μM, respectively.12 These relatively potent inihibitors are selective and non-permanent in their mode of action. The co-crystal structure of domain VI of calpain bound to PD150606 indicated that these inhibitors bound to a novel and possibly unique Ca2+-regulatory site (Fig. 1).13 As these compounds are able to cross cellular membranes, they offer attractive opportunities for the selective inhibition of calpains in living cells.
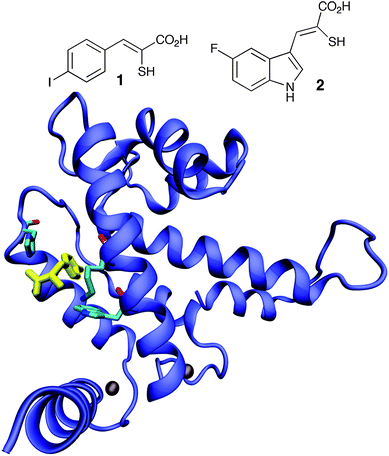 | ||
| Fig. 1 Structures of mercaptoacrylate inhibitors PD150606 (1) and PD151746 (2) and sketch of the structure of 1 bound to domain VI of calpain.13 | ||
We now report the synthesis of a series of monohalide mercaptoacrylic acid derivatives and the structure activity relationship to investigate the efficacy of these molecules for inhibition of Ca2+-activation of calpain-1. This strategy led to the discovery of the most potent cell penetrable calpain-1 inhibitor reported so far.
Synthesis of mercaptoacrylate derivatives
At the outset of this work the intention was to target all possible monohalogenated mercaptoacrylate derivatives of PD150606 and PD151746 with the halogen in each available position of the aromatic ring (Fig. 2).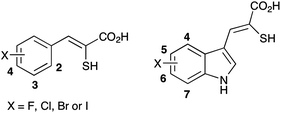 | ||
| Fig. 2 Inhibitors of calpain-1 targeted for synthesis in this study. | ||
The general synthesis of the mercaptoacrylic acid products is outlined in Scheme 1. Aryl aldehyde (3) was condensed with 2-thioxo-4-thiazolidinone (rhodanine) in acetic acid/sodium acetate buffer. The resulting thioxothiazolidinone derivative 4, typically isolated in at least 70% yield, was then subjected to base catalysed hydrolysis and the carboxylic acid liberated by treatment with hydrochloric acid.14 The final compounds were isolated in yields of 50–70%.
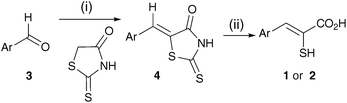 | ||
| Scheme 1 Reagents and conditions: (i) rhodanine, AcOH, NaOAc, 130 °C; (ii) aq. NaOH, 95 °C then aq. HCl, 0 °C.‡ | ||
This synthesis was sufficient for compounds where the halogenated benzaldehyde or indole 3-carboxaldehyde was commercially available. In some cases only the halogenated aromatic ring was available and so the aldehyde was introduced using Vilsmeier–Haack reactions with yields in excess of 80% (see ESI).15 There were four halogenated indoles that required synthesis in this study prior to carrying out the mercaptoacrylic acid synthesis described above; 7-fluoroindole (6a), 7-bromoindole (6b), 6-iodoindole (10) and 4-iodoindole (13).
7-Fluoroindole and 7-bromoindole were synthesised via the Bartoli indole synthesis (Scheme 2).16 1-Fluoro-2-nitrobenzene (5a) and 1-bromo-2-nitrobenzene (5b) were treated with three equivalents of vinylmagnesium bromide to yield the indole ring (42% and 14% for 6a and 6b, respectively).
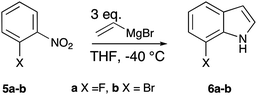 | ||
| Scheme 2 Synthesis of substituted indoles using the Bartoli procedure. | ||
6-Iodoindole (10) was generated in five steps from 6-nitroindoline (7) (Scheme 3).17 Briefly, acylation of the indole nitrogen was followed by reduction of the nitro group using hydrogenation over 10% Pd on activated charcoal in 76% yield over the two steps. The amino group of the resulting indoline derivative 8 was then converted to the iodide in a Sandmeyer reaction and the acyl protecting group removed by base catalysed hydrolysis at 75 °C to generate 6-iodoindoline (9) in 64% yield. Finally, 6-iodoindole (10) was obtained in 77% yield from 9 by air oxidation catalysed by Co(salen)2.18
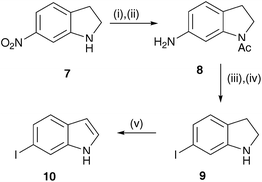 | ||
| Scheme 3 Reagents and conditions: (i) Ac2O, 130 °C; (ii) H2-Pd/C, MeOH; (iii) NaNO2, KI, AcOH, 0 °C; (iv) aq. NaOH, MeOH, 75 °C; (v) Co(salen)2, O2, MeOH. | ||
4-Iodoindole (13) was prepared in two steps from methyl indole-3-carboxylate (11) in 33% overall yield (Scheme 4).19 First, 11 was treated with thallium (III) trifluoroacetate in trifluoroacetic acid. Treatment with potassium iodide displaced thallium to give 12 and the ester was hydrolysed and decarboxylated with NaOH in methanol at 85 °C to yield 13.
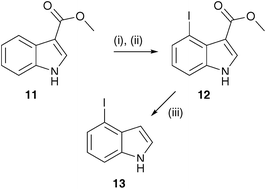 | ||
| Scheme 4 Reagents and conditions: (i) Tl(O2CCF3)3, TFA, 20 °C; (ii) KI, H2O, 20 °C; (iii) NaOH, MeOH, 85 °C. | ||
All indole derivatives were formylated using a Vilsmeier–Haack reaction and converted to the final mercaptoacrylic acid products as described above (ESI). However, 4-iodo-indole derivative of 2 was not accessible by this route; in the final hydrolysis step iodide was lost in an intra-molecular ipso-substitution reaction that led to the formation of the bridged tricyclic indole dervivative 15. A similar reaction also occurred during attempts to prepare the 2-iodo-phenyl analogue of 1 (Scheme 5). These two iodo analogues of 1 and 2 were therefore not assessed in this study although the benzothiophene derivative 17 was analysed as an inhibitor of calpain and found to retain significant albeit reduced potency compared to the mercaptoacrylates themselves (vide infra). It may be noted at this point that mercaptoacrylates of this kind have been used in the past to prepare thiophene derivatives using an oxidative iodination procedure.21
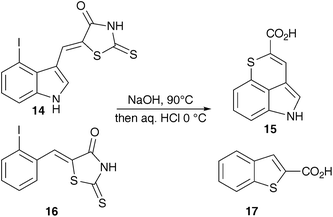 | ||
| Scheme 5 Products from hydrolysis of 2-iodophenyl and 4-iodoindole thioxothiazolidinone. | ||
Assay of inhibitory activity
Two assays for calpain-1 activation were established; the first was based on the cleavage of Suc-Leu-Tyr-7-amino-4-methylcoumarin (SucLY-AMC), a recognised substrate for cysteine proteases,12 while the second used a calpain-specific substrate with a fluorophore (fluorescein) and quencher (DABCYL) linked to either end of an octapetide (H–K(FAM)-EVYGMMK(DABCYL)-OH).20 On cleavage of the peptide, the intensity of the fluorescence increased as the FRET effect of the quencher was relieved. Calpain-1 purified from porcine red blood cells22 in the absence of Ca2+-ions had no effect on either substrate. However, on addition of Ca2+ (0.1 mM), cleavage of the fluorogenic substrates proceeded and the fluorescent signal was monitored continously (Fig. 3). The initial rate, which was proportional to the amount of active enzyme, was taken as a measure of the Ca2+-activated calpain-1 activity in the assay.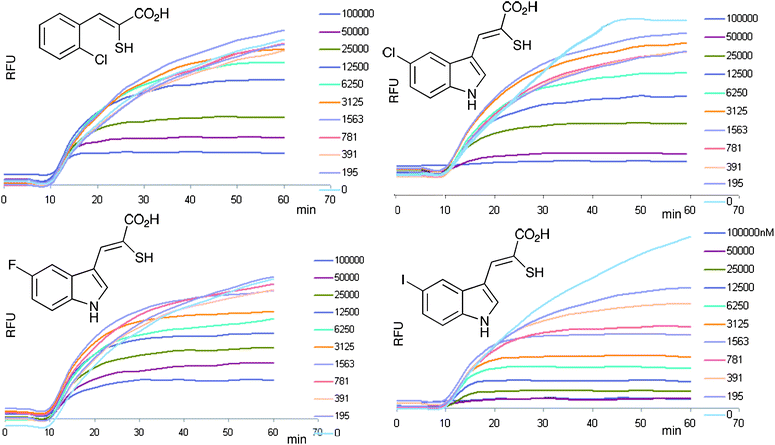 | ||
| Fig. 3 The inhibition of calpain-1 activation. Raw data is shown for typical calpain-1 assays using a fluorogenic FRET-based substrate. The signals show the fluorescence (λex = 490 nm, λ em = 520 nm)20 monitored over 60 min. At 10 min, Ca2+ was added to each assay and the characteristic increase in signal was measured thereafter. Each family of curves shows the effect of increasing concentrations of inhibitor from approximately 100 nM −10 μM. | ||
The 24 monohalide mercaptoacrylate compounds generated here (plus the benzothiophene analogue 17 and the two known inhibitors 1 and 2 for comparison) were tested for their ability to inhibit the Ca2+-activated step in these assays and the concentration of compound required for 50% inhibition (IC50) determined from concentration-inhibition curves (see ESI). The two calpain substrates yielded quantitatively different results, but there was a good correlation between the IC50 values obtained from either substrate. Both assays showed that a number of compounds were highly potent inhibitors of the enzyme (Table 1).
| AMC substrate | IC50 (μM) | |||
|---|---|---|---|---|
| Phenyl | Fluoro | Chloro | Bromo | Iodo |
| 2 | 23.2 | 20.7 | 9.1 | 42.8 a |
| 3 | 31.5 | 13.1 | 8.4 | 23.9 |
| 4 | 5.6 | 15.0 | 1.5 | 5.2 |
| Indole | Fluoro | Chloro | Bromo | Iodo |
| 4 | 0.30 | 0.14 | 0.10 | — |
| 5 | 0.93 | 0.05 | 0.02 | 0.09 |
| 6 | 0.30 | 0.10 | 0.09 | 0.30 |
| 7 | 0.11 | 0.09 | 0.07 | 0.22 |
| FRET substrate | IC50 (μM) | |||
|---|---|---|---|---|
| a Compound actually tested was the benzothiophene derivative 17. | ||||
| Phenyl | Fluoro | Chloro | Bromo | Iodo |
| 2 | 21.9 | 11.9 | 21.0 | 29.6 a |
| 3 | 19.0 | 4.2 | 4.0 | 4.3 |
| 4 | 6.9 | 2.6 | 1.5 | 2.2 |
| Indole | Fluoro | Chloro | Bromo | Iodo |
| 4 | 0.01 | 0.02 | 0.006 | — |
| 5 | 0.80 | 0.25 | 0.006 | 0.10 |
| 6 | 0.01 | 0.21 | 0.007 | 0.17 |
| 7 | 0.12 | 0.01 | 0.002 | 0.09 |
Halides in positions 4 of the phenyl ring and position 7 of the indole ring generated the most effective inhibitors. The indole compounds were significantly more effective than the phenyl derivatives; there was a good correlation between the size of the halide and its efficacy: I ≈ Br > Cl > F. The most effective inhibitors were indole compounds with iodine or bromine at position 7.
The compounds showing the greatest potency for inhibition of calpain-1 were tested as inhibitors of the calpain-1 dependent process of cell “spreading”. Human neutrophils were isolated from blood according to established procedures23 and incubated either with solvent (DMSO, 0.1% max) or compounds (dissolved in DMSO) before being placed on a glass slide for quantitation of the rate of spreading. Neutrophils were allowed to sediment onto a glass coverslip held at 37 °C, while microscopic images were acquired of a single random field focussed at the plane of the glass surface. In this way, the process of cell spreading was captured for individual cells from the moment of contact with the surface and during their adhesion and increase in cell area (spreading). Images were analysed at 1 min intervals by processing with Image J software (NIH) to define the cell edge before quantifying the area occupied by the cells at each time point. This assay was used to establish whether the inhibitors gained access to the inside of cells of clinical relevance. Neutrophil spreading also provides a surrogate indicator of the extent of calpain activation in these cells.24,25 Spontaneous cytosolic Ca2+ rises, detectable using fluo4,26 are triggered in individual cells before they spread.26,27 This persisted in cells treated with inhibitors indicating that the inhibitory step was “down stream” of the Ca2+-signal and consistent with calpain specific action. It should be noted that it was not possible to totally abolish cell spreading with these inhibitors, but this may be because the spontaneous spreading process probably also involves other pathways along with the Ca2+-dependent pathways.28 However, both the rate and extent of cell spreading were significantly impaired by the novel compounds (Fig. 4).
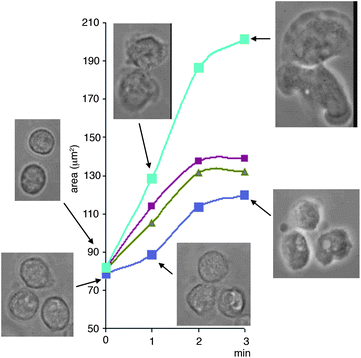 | ||
| Fig. 4 Inhibition of neutrophil spreading by the calpain-1 inhibitors reported here. The graph shows the area of neutrophils immediately in contact with the glass surface and the change over the subsequent 3 min. The images are from a typical experiment, in which the same field of cells are shown at the time points indicated. This was representative of experiments with cells from at least 3 individual donors, where cell fields were selected at random and at least 50 cells were analysed. The upper images are for cells incubated with DMSO (0.1%) and the lower curves are for cells incubated with inhibitors having the following halides at position 6 on the indole ring of inhibitor series 2 (top to bottom) F, Cl and Br (10 μM). | ||
Conclusions
This investigation shows that the mercaptoacrylates previously reported as calpain inhibitors12 are members of a larger family of inhibitors. By systematic synthesis, a structure–activity relationship has revealed that the larger halogens Br and I at position 4 of the phenyl derivatives and position 7 of the indole derivatives were associated with the more potent inhibitors. The bromo-indole derivatives are the most potent calpain inhibitors prepared to date, inhibiting calpain-1 with IC50 values in the low nanomolar range according to the more calpain-specific FRET assay. Furthermore, despite a previous report that suggested that both the carboxylate and thiolo functionalities were crucial to calpain inhibition, it was found that the benzothiophenyl carboxylate 17 retained some ability to inhibit calpain (Table 1). This compound is more stable than the mercaptoacrylates in solution due to the loss of the SH group and so may prove of interest in developing further series of calpain inhibitors with improved pharmacokinetic stability. In this regard it should be noted that the final mercaptoacrylate compounds prepared in this study were all found to be suspectible to air-oxidation in the presence of water and form a disulfide. The HPLC retention time of each compound was observed to change over a period of approximately 3 h in the presence of water. Isolation of this new material and analysis by 1H NMR spectroscopy showed no SH resonance at δH ∼ 5.30 ppm and concomitant shifts in resonances of aromatic CH-protons. The disulfide was readily reduced back to the thiolo form through addition of reducing agents such as tris(2-carboxyethyl)phosphine (TCEP) or dithiothreitol (DTT) (see ESI for supporting data). All assays were performed under reducing conditions (10 mM DTT, ESI) sufficient to rapidly reduce (<1 min) any disulfide formed. Assays carried out with preformed disulfides showed identical results to those carried out on analogues in the reduced form.These novel compounds were also effective at blocking calpain-dependent neutrophil cell-spreading. This in vitro cellular event is a model for the behaviour of neutrophils, which occurs in vivo during extravasation, when circulating neutrophils adhere and spread onto the endothelial cell lining of the blood vessels.29 Inhibition of this step would thus limit the rate at which neutrophils leave the blood and enter the inflamed tissues, and so supress inflammation. Although inhibition of this step can be achieved by some anti-inflammatory “biologics” eg anti-TNF antibody,30 these are usually adminstered by intravenous infusion and there are significant cost implications in their use. There is thus a clear need for effective small molecular weight inhibitors of the process which can, ideally, be taken orally. The compounds reported in this paper represent a significant step towards achieving that goal. In addition, they form the basis for a rational approach to generating small molecular weight anti-calpain reagents that have the potential to be used therapeutically for the treatment of inflammatory disease.
This work was supported by the U.K.'s Engineering and Physical Sciences Research Council (EPSRC) and the Medical Research Council (MRC) through grant G0701192. We are grateful to the EPSRC national mass spectrometry service at Swansea for mass spectral analyses.
Notes and references
- Y. H. Huang and K. K. W. Wang, Trends Mol. Med., 2001, 7, 355–362 Search PubMed.
- H. Sorimachi, S. Ishiura and K. Suzuki, Biochem. J., 1997, 328, 721–732 Search PubMed.
- B. Todd, D. Moore, C. C. S. Deivanayagam, G. D. Lin, D. Chattopadhyay, M. Maki, K. K. W. Wang and S. V. L. Narayana, J. Mol. Biol., 2003, 328, 131–146 Search PubMed.
- A. Glading, D. A. Lauffenburger and A. Wells, Trends Cell Biol., 2002, 12, 46–54 Search PubMed.
- C. M. Hosfield, J. S. Elce, P. L. Davies and Z. C. Jia, EMBO J., 1999, 18, 6880–6889 Search PubMed.
- E. Dainese, R. Minafra, A. Sabatucci, P. Vachette, E. Melloni and I. Cozzani, J. Biol. Chem., 2002, 277, 40296–40301 Search PubMed.
- K. K. W. Wang, Trends Neurosci., 2000, 23, 20–26 Search PubMed.
- K. Croce, R. Flaumenhaft, M. Rivers, B. Furie, B. C. Furie, I. M. Herman and D. A. Potter, J. Biol. Chem., 1999, 274, 36321–36327 Search PubMed.
- M. Azam, S. S. Andrabi, K. E. Sahr, L. Kamath, A. Kuliopulos and A. H. Chishti, Mol. Cell. Biol., 2001, 21, 2213–2220 Search PubMed.
- H. A. Menard and M. ElAmine, Immunol. Today, 1996, 17, 545–547 Search PubMed.
- I. O. Donkor, Curr. Med. Chem., 2000, 7, 1171–1188 Search PubMed.
- K. K. W. Wang, R. Nath, A. Posner, K. J. Raser, M. Buroker-Kilgore, I. Hajimohammadreza, A. W. Probert, F. W. Marcoux, Q. H. Ye, E. Takano, M. Hatanaka, M. Maki, H. Caner, J. L. Collins, A. Fergus, K. S. Lee, E. A. Lunney, S. J. Hays and P. W. Yuen, Proc. Nat. Acad. Sci. USA, 1996, 93, 6687–6692 Search PubMed.
- H. Blanchard, P. Grochulski, Y. Li, J. S. C. Arthur, P. L. Davies, J. S. Elce and M. Cygler, Nat. Struct. Biol., 1997, 4, 532–538 Search PubMed.
- K. K. Wang and P.-W. Yuen, 1996, US patent no. 5554767.
- I. M. Downie, M. J. Earle, H. Heaney and K. F. Shuhaibar, Tetrahedron, 1993, 49, 4015–4034 CrossRef CAS.
- G. Bartoli, G. Palmieri, M. Bosco and R. Dalpozzo, Tetrahedron Lett., 1989, 30, 2129–2132 CrossRef CAS.
- Y. Yamada, A. Akiba, S. Arima, C. Okada, K. Yoshida, F. Itou, T. Kai, T. Satoua, K. Takeda and Y. Harigaya, Chem. Pharm. Bull., 2005, 53, 1277–1290 Search PubMed.
- A. L. Borror, E. Chinoporos, M. P. Filosa, S. R. Herchen, C. P. Petersen and C. A. Stern, J. Org. Chem., 1988, 53, 2047–2052 CrossRef CAS.
- J. B. Hendrickson and J. Wang, Org. Lett., 2004, 6, 3–5 CrossRef.
- S. Mittoo, L. E. Sundstrom and M. Bradley, Anal. Biochem., 2003, 319, 234–238 CrossRef CAS.
- P. M. Chakrabarti, N. B. Chapman and K. Clarke, Tetrahedron, 1969, 25, 2781–2785 Search PubMed.
- A. Kitahara, T. Sasaki, T. Kikuchi, N. Yumoto, N. Yoshimura, M. Hatanaka and T. Murachi, J. Biochem., 1984, 95, 1759–1766 Search PubMed.
- E. J. Pettit and M. B. Hallett, J. Cell Sci., 1996, 109, 1689–1694 Search PubMed.
- S. Dewitt and M. Hallett, J. Leuk. Biol., 2007, 81, 1160–1164 Search PubMed.
- M. B. Hallett and S. Dewitt, Trends Cell Biol., 2007, 17, 209–214 CrossRef CAS.
- P. W. Marks and F. R. Maxfield, J. Cell Biol., 1990, 110, 43–52 CrossRef CAS.
- B. A. Kruskal, S. Shak and F. R. Maxfield, Proc. Nat. Acad. Sci. USA, 1986, 83, 2919–2923 Search PubMed.
- E. J. Pettit and M. B. Hallett, J. Cell Sci., 1998, 111, 2209–2215 Search PubMed.
- K. Ley, C. Laudanna, M. I. Cybulsky and S. Nourshargh, Nat. Rev. Immunol., 2007, 7, 678–689 CrossRef CAS.
- J. G. Stoll and U. Yasothan, Nat. Rev. Drug Discov., 2009, 8, 693–694 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of the synthesis and stability studies of mercaptoacrylates, calpain inhibition assays and neutrophil spreading assays. See DOI: 10.1039/c2md00280a |
| ‡ A Z-configuration for the final compounds is assumed throughout. This was not determined in this study but is ubiquitous in all previous literature.12,14 |
| This journal is © The Royal Society of Chemistry 2012 |
