Studying the differential co-expression of microRNAs reveals significant role of white matter in early Alzheimer's progression†
Abstract
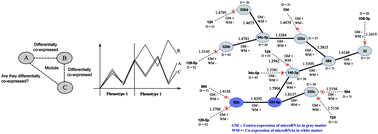
MicroRNAs (miRNAs) are a class of short non-coding RNAs, which show tissue-specific regulatory activity on genes. Expression profiling of miRNAs is an important step for understanding the pathology of Alzheimer's disease (AD), a neurodegenerative disorder originating in the brain. Recent studies highlight that miRNAs enriched in gray matter (GM) and white matter (WM) of AD brains show differential expression. However, no in-depth study has yet been conducted on analysing the differential co-expression of pairs of miRNAs over GM and WM. Two genes (or miRNAs) are said to be co-expressed if their expression profiles change similarly over a number of samples. A pair of co-expressed genes under a condition type (or phenotype) may not remain co-expressed, or get contra-expressed, under another condition. Such pairs of genes are referred to as differentially co-expressed. Such an investigation in the early stage of AD is reported in this article. A network of differentially co-expressed miRNAs in GM and WM is first built. Analysis of the differential co-expression property reveals that such a network can not have any cycle. We use the notion of switching to distinguish two distinct types of differential co-expression patterns – a pair of miRNAs that are highly co-expressed in GM but does not remain so in WM, and vice versa. Based on this, we find the substructures, referred to as differentially co-expressed switching tree (DCST), that throughout have similar pattern of switching. The miR-423-5p emerges as a hub of the network. We extract subtrees of these DCSTs that have similar switching pattern throughout. These substructures are found to be both statistically and biologically significant. A large number of miRNAs obtained from the DCSTs are found to have association with AD, most of which are enriched in WM. This computational study therefore indicates a significant role of WM in early AD progression, a hitherto less acknowledged fact.

 Please wait while we load your content...
Please wait while we load your content...