Surface acoustic wave (SAW) acoustophoresis: now and beyond
Sz-Chin Steven
Lin
a,
Xiaole
Mao
b and
Tony Jun
Huang
*a
aDepartment of Engineering Science and Mechanics, The Pennsylvania State University, University Park, PA 16802, USA. E-mail: junhuang@psu.edu
bThe Procter and Gamble Company, Mason, Ohio 45040, USA
First published on 10th July 2012
Abstract
On-chip manipulation of micro-objects has long been sought to facilitate fundamental biological studies and point-of-care diagnostic systems. In recent years, research on surface acoustic wave (SAW) based micro-object manipulation (i.e., SAW acoustophoresis) has gained significant momentum due to its many advantages, such as non-invasiveness, versatility, simple fabrication, easy operation, and convenient integration with other on-chip units. SAW acoustophoresis is especially useful for lab-on-a-chip applications where a compact and non-invasive biomanipulation technique is highly desired. In this Focus article, we discuss recent advancements in SAW acoustophoresis and provide some perspectives on the future development of this dynamic field.
Introduction
The ability to manipulate micro-objects (such as cells, micro-particles, and droplets) in clearly defined patterns and paths is critical for a wide variety of lab-on-a-chip applications. Historically, optical tweezers1 have been the primary tool used in the scientific community for micro-object manipulation. Despite the remarkable capability and success, optical tweezers have notable limitations: complex and bulky instrumentation, high equipment costs, potential damage to cells, among others.2 In the past decades, the emergence of lab-on-a-chip technology has motivated significant effort to replace optical tweezers. Many alternative manipulation methods, such as dielectrophoresis (DEP),3 magnetic tweezers,4 and optoelectronic tweezers,5 have been proposed and have their own advantages.More recently (in the past five years or so), surface acoustic wave (SAW) based micro-object manipulation has become a vibrant research field.6–11 A SAW is a type of acoustic wave that propagates along the surface of an elastic material with the majority of acoustic energy confined within one wavelength of the surface. SAW devices require simple photolithography processes to fabricate interdigitated metallic electrodes on piezoelectric substrates. Furthermore, SAW properties can be tuned through applied electric signals which provide much simpler control than most alternative techniques. Most importantly, ultrasound at appropriate intensities has been proven safe to biological samples and is widely used in biomedical imaging and diagnostics.
When encountering a microliter droplet, SAWs generate a body force on the droplet and move it along the wave propagation direction. In addition, by manipulating the acoustic waves themselves, pressure nodes in a standing SAW field can be created and controlled to manoeuvre cells or other bioparticles in a programmed manner. We call these SAW-based micro-object manipulation approaches SAW acoustophoresis—acoustophoresis being defined as migration with sound. SAW acoustophoresis provides many advantages, such as simple design and fabrication, low cost, compact device, low power consumption, non-invasiveness, and convenient integration with other on-chip units, all of which made it an important progression in non-invasive micromanipulation technology toward on-chip applications. Compared with bulk acoustic wave (BAW) based acoustophoresis, SAW acoustophoresis allows one to better control a wider range of excitation frequencies—by simply adjusting the period of inter-digital transducers (IDTs)—and utilize higher excitation frequencies (when needed). As a result, SAW acoustophoresis can be more versatile and can achieve finer resolution in particle manipulation. In this Focus article, we will discuss the most recent advances in SAW acoustophoresis and our perspectives for future developments.
SAW-based droplet manipulation
SAW acoustophoresis was introduced in a study of the interaction between SAWs and a microliter droplet sitting on top of the SAW propagating surface. SAWs are normally generated by imposing an electrical signal on the metallic IDTs fabricated on top of a piezoelectric substrate. The central frequency of the SAWs is determined by the period of the IDTs, while its bandwidth and directionality are regulated by the number of the electrodes and aperture (overlapping length of the electrodes) of the IDTs, respectively. Lithium niobate (LiNbO3) is the most commonly used piezoelectric material in SAW acoustophoresis due to its excellent electromechanical coupling, biocompatibility, and optical transparency. As SAWs travel and reach the boundary of the droplet, part of their energy is absorbed by the fluid and reradiates in the form of longitudinal waves, which in turn actuate a bulk fluid flow within the droplet and lead to internal streaming (Fig. 1A).12 By changing the incident location of the SAW, one can use the induced symmetric and asymmetric acoustic streaming to achieve a variety of droplet-based applications, including rapid fluid mixing, particle concentration, particle patterning, and reorientation of nano-objects without significantly deforming the shape of the droplet. At stronger SAW amplitudes, the droplet can be pushed by the induced body force to move along the direction of SAW propagation. At extremely high acoustic power, however, the leakage energy oscillates the whole droplet rapidly and causes jetting and atomization of the fluid, which can find uses in applications such as nano-printing and nanoparticle generation for drug delivery. | ||
| Fig. 1 (A) A schematic showing the generation, propagation, and reradiation of SAW. The enclosed patterns inside the droplet are the simulated acoustic streaming. (B) A representative design of SAW-based droplet transportation devices showing translation and merging of droplets. The droplets are approximately 100 nl each. (C) Collection of 10 μm melamine particles.16 (D) Removal of nonspecifically bound proteins by SAW-induced acoustic streaming. Images reproduced from ref. 12, 14, and 17 with permission from IOP, Elsevier, and APS publishing. | ||
Currently, most microfluidic devices are not truly “Lab-on-a-Chip” systems in the sense that they rely heavily on off-chip, fluid-driven components (e.g., syringe pumps, switches, and pressure sources) that are yet to be effectively miniaturized. In this regard, SAW-driven droplet manipulation can overcome such limitations—it can effectively transport minute volumes of sample reagents through the control of electric signals that are generated by on-chip integrated circuits, rather than relying on bulky, off-chip components. As a result, SAW-driven droplet manipulation is perfect for compact, fully integrated “Lab-on-a-Chip” systems and are particularly useful in point-of-care diagnostic systems for remote regions and the developing world.13Fig. 1B shows a representative design of such SAW-based droplet transportation devices, which has six independent pairs of IDTs to achieve droplet transportation in two dimensions.14 Microliter to sub-microliter droplets of different reagents can be acoustically moved and merged to perform various chemical reactions in a controllable fashion. The reported speed of a microliter droplet actuated with SAWs has reached as high as 10 cm s−1, at least one order of magnitude higher than the speed of any other droplet microfluidic technologies.8 With its advantages in high speed, easy and programmable operation, and simplicity in fabrication, SAW-based droplet transportation is an ideal approach for the development of on-chip automated chemical synthesis and analysis systems. For example, by moving a DNA-encapsulated droplet between heaters and sinks, on-chip polymerase chain reaction (PCR) was achieved with a volume as low as 200 nl.15
SAW-driven droplet translation can also be employed to collect micro/nano particles along the droplet's transportation path. Fig. 1C shows the sequential images of the SAW-based particle collection.16 As the droplet is propelled by the acoustic energy, the SAW-induced acoustic streaming inside the droplet lifts the particles from the surface to follow the flow circulation. As a result, rapid and efficient particle collection/removal can be obtained by electronically controlling SAW power and the path of the droplet. A similar principle is applied to remove non-specifically bound (NSB) proteins from biosensors to improve their sensitivity.17,18 The fluorescent images in Fig. 1D depict the effectiveness of the removal of NSB proteins. Researchers have also proved that SAWs do not inhibit the activity of a bound protein, thus rendering it a biocompatible technique. Because the LiNbO3 substrate is transparent, SAW-based droplet manipulation devices are compatible with optical characterization—they can be directly placed under a microscope to monitor the reaction in real-time. In this regard, SAW-based droplet manipulation devices can be seamlessly integrated with miniaturized portable optical detection units (e.g., CCD cameras) to build a micro total analysis system (μTAS) for point-of-care medical diagnostics. At the current stage, compared to other droplet microfluidic systems,19 SAW-based microliter droplet manipulation techniques have relatively low throughput—a limitation that will need to be addressed in the future.
SAW-based single cell manipulation
In many biological studies where cell–cell, cell–biomolecule, and cell–environment interactions are involved, a non-invasive, dexterous single cell manipulation technique is needed. Optical tweezers have been widely considered as the most powerful single cell manipulation tool; however, optical tweezers require a complex and expensive optical setup, which limit their accessibility to many research teams. Additionally, focused laser-induced heating could potentially cause permanent physiological damage to biological objects.2 As a result, researchers have recently been focusing on developing SAW-based single cell manipulation platforms to provide a more non-invasive, accessible tool for single cell manipulation.In order to manipulate a cell using SAW acoustophoresis, one must first acoustically trap the cell in the suspending medium. As introduced in the previous section, the interaction between a traveling SAW and a fluid medium results in acoustic streaming which moves suspended objects along the fluid circulation; this circulation makes it challenging to trap objects at designated locations. However, it has been established that objects in a standing acoustic field will experience an acoustic radiation force that pushes them toward the pressure nodes or anti-pressure nodes of the standing field, depending on the elastic properties of the object and the surrounding medium.20,21 For most biological objects in aqueous solutions, they will be trapped at pressure nodes. Using this mechanism, one can generate a standing SAW field using pairs of aligned IDTs to pattern cells in one or two dimensions. Fig. 2A shows a schematic of the device and the simulated pressure distribution in one- and two-dimensional standing SAW fields. Patterning a variety of cells or proteins into linear or rectangular arrays in a stationary microfluidic chamber has been demonstrated and is valuable in applications such as tissue engineering and regenerative medicine. The processing time is normally on the order of seconds, and the period is determined by the SAW wavelength which is defined by the pitch of the IDTs. Recently it has been demonstrated that by changing the IDTs from regular straight ones to slanted finger interdigital transducers (SFIT), the distance between cells can be tuned on a single chip (Fig. 2B).11 Tunable control of the physical distance between cells facilitates many on-chip cell studies such as dynamic control of cell–cell interactions.
 | ||
| Fig. 2 (A) A schematic showing the generation of a standing SAW field. The bottom images show the simulated 1D and 2D pressure fields. (B) Tunable patterning of HL-60 human promyelocyte leukemia cells. I, II, and III show that the period of 1D cell pattern can be adjusted on the same device: (I) 150 μm; (II) 78 μm; and (III) 60 μm.11 | ||
After cells are acoustically trapped at the pressure nodes of a standing SAW field, the next important task is to transport them in a programmable fashion. It is straightforward that the trapped cells can be moved by moving the location of the pressure nodes. There are two ways to achieve this goal: changing the phase or wavelength of each generated SAW. Recent studies have demonstrated that the phase of a generated SAW can be precisely adjusted by a voltage-controlled phase shifter.22,23 Therefore, cells trapped at the pressure nodes of a standing field can be shifted toward one side of the transducers. Ding et al. recently presented a design that can change the wavelength of the excited SAW using chirp IDTs (Fig. 3A).24 A chirp IDT has a linear gradient in the pitch that allows it to resonate at a wide range of frequencies, thus it is able to excite SAWs of different wavelengths by tuning the electric signals. Fig. 3B shows that a single object (microparticle, cell, and organism) can be moved in a programmable manner in two dimensions by electronically controlling the standing SAW field. Arbitrary control of single micro-objects in two-dimensions is a major goal for micromanipulation in general; hence such a SAW device is coined as “acoustic tweezers.” Cell viability and proliferation studies showed the non-invasiveness of the acoustic tweezers.24 The non-invasiveness, versatility, compactness, simplicity in design, and ease of operation render the acoustic tweezers a strong competitor to its optical counterpart: optical tweezers.
 | ||
| Fig. 3 (A) Device structure and working mechanism of tunable SAW-based acoustic tweezers. (B) Two-dimensional manipulation of single particle, cell, and organism. (I) Stacked images used to demonstrate independent motion in x and y using a 10 μm fluorescent polystyrene bead to write the word “PNAS”. (II) Stacked images showing dynamic control of a bovine red blood cell to trace the letters “PSU”. (III) A single organism (C. elegans) was transported in two dimensions. An optical image of C. elegans (IV) before and (V) after being fully stretched by the acoustic force. Images reproduced from ref. 24. | ||
At the current stage of development, acoustic tweezers have limitations as well. First of all, they have difficulties selecting an individual cell from a group. Second, the current designs cannot manipulate sub-micron objects such as single DNA or protein molecules. While the second limitation may soon be overcome (e.g., by increasing the SAW frequency to generate a stronger force on nano-objects), overcoming the first limitation appears to be a tough task. To make acoustic tweezers as powerful as optical tweezers, new outside-the-box ideas need to be explored, systematic fundamental studies conducted, and system development and integration need to be undertaken. To begin with, the acoustic radiation force model used to calculate the force magnitude needs to be modified to accurately represent the setting in a microfluidic chamber. For example, objects were assumed to be spherical and much smaller than acoustic wavelength; red blood cells have bi-concave shapes and cannot be accurately described by the model. Furthermore, SAW-induced acoustic streaming plays a major role in manipulating objects smaller than a few microns.25–27 It has to be considered together with the acoustic radiation forces to appropriately explain the complex phenomena inside the microfluidic chamber. A multi-physics model that includes the piezoelectric, acoustic streaming, and acoustic scattering effects needs to be developed. To address these challenges, close collaboration between theorists and experimentalists is vital.
Perspectives
SAW-based microliter droplet and single cell manipulation have demonstrated great potential to assist the development of simpler, yet more powerful platforms for a variety of lab-on-a-chip applications. Besides the aforementioned directions, we believe that the further development and implementation of the following concepts will be extremely helpful. First, the disposable superstrate concept is promising in SAW acoustophoresis.28Fig. 4A shows that SAW can be effectively coupled to the superstrate where the droplets or the microfluidic channel are deposited. In this manner, the relatively more expensive piezoelectric substrate can be reused repeatedly, and the superstrate has the freedom to use any materials such as cell culture plates or semiconductor-compatible materials. Second, phononic crystals (PCs) can help gain better control on acoustic wave propagation in SAW acoustophoresis. PCs are engineered periodic structures that can block, bend, and focus an acoustic beam.29–34Fig. 4B shows an example of combining the PC and superstrate concepts to build a reusable fluid jetting device. Finally, system integration will need to be conducted to fulfil the potential of SAW acoustophoresis. SAW devices can be seamlessly integrated with other on-chip components such as multi-dimensional microfluidic channels and optical detection modules to build compact, inexpensive, energy-saving lab-on-a-chip systems. This is an advantage that optical tweezers and some biomanipulation techniques do not possess.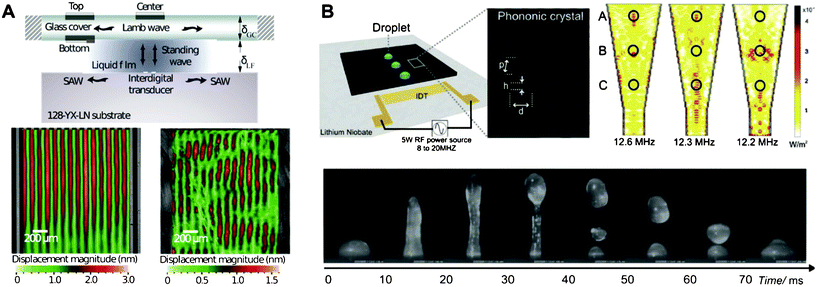 | ||
| Fig. 4 (A) Laser doppler vibrometer scan of the SAW on (left) the substrate and (right) the superstrate showing effective coupling of acoustic energy. (B) Images showing the use of phononic crystals on a superstrate to manipulate droplets. Images reproduced from ref. 28 and 29 with permission from AIP and Wiley publishing. | ||
Conclusions
As we have witnessed, SAW acoustophoresis has become an extremely dynamic and exciting field in recent years. To date, the lab-on-a-chip community has extensively explored and utilized the physics of light (e.g., optical tweezers), electrokinetics (e.g., DEP), and magnetism (i.e., magnetic tweezers) in a variety of ways. Acoustics, especially SAWs, although arriving relatively late in the game, have demonstrated remarkable potential in a very short period of time. We are extremely excited to witness this progress and look forward to seeing more breakthroughs and exciting applications of SAW acoustophoresis.Acknowledgements
This research was supported by the National Institutes of Health (NIH) Director's New Innovator Award (1DP2OD007209-01). We thank Brian Kiraly for helpful discussion.References
- A. Ashkin, J. M. Dziedzic, J. E. Bjorkholm and S. Chu, Opt. Lett., 1986, 11, 288 CrossRef CAS.
- M. B. Rasmussen, L. B. Oddershede and H. Siegumfeldt, Appl. Environ. Microbiol., 2008, 74, 2441–2446 CrossRef CAS.
- S. Park, Y. Zhang, T.-H. Wang and S. Yang, Lab Chip, 2011, 11, 2893–2900 RSC.
- A. Snezhko and I. S. Aranson, Nat. Mater., 2011, 10, 698–703 CrossRef CAS.
- A. Jamshidi, P. J. Pauzauskie, P. J. Schuck, A. T. Ohta, P.-Y. Chiou, J. Chou, P. Yang and M. C. Wu, Nat. Photonics, 2008, 2, 86–89 CrossRef.
- J. Shi, X. Mao, D. Ahmed, A. Colletti and T. J. Huang, Lab Chip, 2008, 8, 221–223 RSC.
- J. Shi, D. Ahmed, X. Mao, S.-C. S. Lin, A. Lawit and T. J. Huang, Lab Chip, 2009, 9, 2890–2895 RSC.
- L. Y. Yeo and J. R. Friend, Biomicrofluidics, 2009, 3, 012002 CrossRef.
- J. Shi, S. Yazdi, S.-C. S. Lin, X. Ding, I.-K. Chiang, K. Sharp and T. J. Huang, Lab Chip, 2011, 11, 2319–2324 RSC.
- J. Nam, H. Lim, D. Kim and S. Shin, Lab Chip, 2011, 11, 3361–3364 RSC.
- X. Ding, J. Shi, S.-C. S. Lin, S. Yazdi, B. Kiraly and T. J. Huang, Lab Chip, 2012, 12, 2491–2497 RSC.
- M. Alghane, B. X. Chen, Y. Q. Fu, Y. Li, J. K. Luo and A. J. Walton, J. Micromech. Microeng., 2011, 21, 015005 CrossRef.
- X. Mao and T. J. Huang, Lab Chip, 2012, 12, 1412–1416 RSC.
- A. Wixforth, Superlattices Microstruct., 2003, 33, 389–396 CrossRef CAS.
- Z. Guttenberg, H. Müller, H. Habermüller, A. Geisbauer, J. Pipper, J. Felbel, M. Kielpinski, J. Scriba and A. Wixforth, Lab Chip, 2005, 5, 308–317 RSC.
- M. K. Tan, J. R. Friend and L. Y. Yeo, Lab Chip, 2007, 7, 618–625 RSC.
- S. K. R. S. Sankaranarayanan, S. Cular, V. R. Bhethanabotla and B. Joseph, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2008, 77, 066308 CrossRef.
- S. Cular, D. W. Branch, V. R. Bhethanabotla, G. D. Meyer and H. G. Craighead, IEEE Sens. J., 2008, 8, 314–320 CrossRef CAS.
- M. T. Guo, A. Rotem, J. A. Heyman and D. A. Weitz, Lab Chip, 2012, 12, 2146–2155 RSC.
- A. Lenshof, C. Magnusson and T. Laurell, Lab Chip, 2012, 12, 1210–1223 RSC.
- C. R. P. Courtney, C.-K. Ong, B. W. Drinkwater, P. D. Wilcox, C. Demore, S. Cochran, P. Glynne-Jones and M. Hill, J. Acoust. Soc. Am., 2010, 128, E195–E199 CrossRef.
- N. D. Orloff, J. R. Dennis, M. Cecchini, E. Schonbrun, E. Rocas, Y. Wang, D. Novotny, R. W. Simmonds, J. Moreland, I. Takeuchi and J. C. Booth, Biomicrofluidics, 2011, 5, 044107 CrossRef.
- L. Meng, F. Cai, Z. Zhang, L. Niu, Q. Jin, F. Yan, J. Wu, Z. Wang and H. Zheng, Biomicrofluidics, 2011, 5, 044104 Search PubMed.
- X. Ding, S.-C. S. Lin, B. Kiraly, H. Yue, S. Li, I.-K Chiang, J. Shi, S. J. Benkovic and T. J. Huang, Proc. Natl. Acad. Sci. U. S. A., 2012 DOI:10.1073/pnas.1209288109.
- M. K. Tan, J. R. Friend, O. K. Matar and L. Y. Yeo, Phys. Fluids, 2010, 22, 112112 CrossRef.
- H. Bruus, Lab Chip, 2012, 12, 20–28 RSC.
- M. Wiklund, R. Green and M. Ohlin, Lab Chip, 2012, 12, 2438–2451 RSC.
- R. P. Hodgson, M. K. Tan, L. Y. Yeo and J. R. Friend, Appl. Phys. Lett., 2009, 94, 024102 CrossRef.
- Y. Bourquin, R. Wilson, Y. Zhang, J. Reboud and J. M. Cooper, Adv. Mater., 2011, 23, 1458–1462 CrossRef CAS.
- R. Wilson, J. Reboud, Y. Bourquin, S. L. Neale, Y. Zhang and J. M. Cooper, Lab Chip, 2011, 11, 323–328 RSC.
- J. Reboud, R. Wilson, Y. Zhang, M. H. Ismail and J. M. Cooper, Lab Chip, 2012, 12, 1268–1273 RSC.
- J. Shi, S.-C. S. Lin and T. J. Huang, Appl. Phys. Lett., 2008, 92, 111901 CrossRef.
- S.-C. S. Lin, T. J. Huang, J.-H. Sun and T.-T. Wu, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 094302 CrossRef.
- S.-C. S. Lin and T. J. Huang, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 174303 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
