Microfluidic synthesis of multifunctional Janus particles for biomedical applications
Shikuan
Yang
a,
Feng
Guo
a,
Brian
Kiraly
a,
Xiaole
Mao
ab,
Mengqian
Lu
a,
Kam W.
Leong
c and
Tony Jun
Huang
*ab
aDepartment of Engineering Science and Mechanics, The Pennsylvania State University, University Park, PA 16802, USA
bDepartment of Bioengineering, The Pennsylvania State University, University Park, PA 16802, USA. E-mail: junhuang@psu.edu
cDepartment of Biomedical Engineering, Duke University, CIEMAS 1395, PO Box 90281, Durham, NC 27780, USA
First published on 14th May 2012
Abstract
Multifunctional Janus particles have a variety of applications in a wide range of fields. However, to achieve many of these applications, high-throughput, low-cost techniques are needed to synthesize these particles with precise control of the various structural/physical/chemical properties. Microfluidics provides a unique platform to fabricate Janus particles using carefully controlled liquid flow in microfluidic channels to form Janus droplets and various types of solidification methods to solidify them into Janus particles. In this Focus article, we summarize the most recent representative works on Janus particle fabrication in microfluidics. The applications of Janus particles in biomedical areas are emphasized. We believe that microfluidics-enabled multifunctional Janus particles could resolve multiple prevalent issues in biomedicine (e.g., disease monitoring at an early stage, high-throughput bioassays, therapeutic delivery) if persistent effort and collaboration are devoted to this direction.
Introduction
Micro/nanostructures have attracted tremendous attention because of their fascinating properties and wide applications in electronics, magnetics, sensing, optics, and nanomedicine.1–3 The past decades have witnessed the rapid development of micro/nanostructure fabrication through wet-chemistry based techniques which carefully control the nucleation and growth processes.4,5 Recent demands for increased functionality, however, have shed light on the limitations of conventional micro/nanostructures. For these complex applications, multifunctional structures are sought to meet the diverse requirements from different disciplines. One of these structures is the Janus particle, first coined by P. G. de Gennes in his Nobel Prize address. He borrowed the name “Janus” from the Roman God with two heads placed back to back,6 and the particle has quickly grown as a new member in the colloidal family. Strictly speaking, a Janus particle should have two areas with different chemistry, polarity, functionalization and/or other properties with roughly equal surface area (Fig. 1a).7,8 However, the term has been expanded to encompass other multi-segment structures with regions of different composition co-existing in asymmetric geometries (Fig. 1).8 In this Focus article, we consider all multicomponent particles as Janus particles for simplicity (as summarized in Fig. 1). Janus particles possess wide applications in optics, magnetics, plasmonics, colloidal chemistry, and biomedicine due to their fascinating properties.9–13 Currently, however, significant challenges exist in the fabrication and preparation of these Janus particles. The most common methods, including emulsions,14 site-specific growth in solution phase,15 metal evaporation on patterned surfaces,16 and controlled interfacial reactions,17 usually suffer from drawbacks like poor structural control, extremely low production rates, and ineffectiveness in generating complex structures.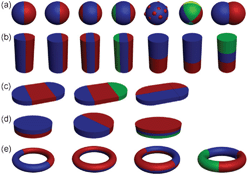 | ||
| Fig. 1 Schematic illustration of the (a) zero- , (b) one- and (c, d, e) two-dimensional Janus particles. Different colours represent different components. | ||
Due to the unique behaviour of fluids in the micrometer scale, microfluidic systems offer many unique characteristics that do not exist in traditional batch processes, including high heat and mass transfer rates, short mixing time scales, short residence times, well-defined reaction temperatures, and high throughput.18 Recently, microfluidics has proven to be an effective platform for Janus particle generation, and a particularly promising platform for synthesizing Janus particles that are difficult (if not completely impossible) to synthesize in batch reactions.18 Here, we first discuss the advantages of microfluidics for the synthesis of Janus particles, then give a few examples of existing and potential applications of Janus particles in biomedicine, and conclude with some perspectives for future of microfluidic synthesis of Janus particles.
Janus particle synthesis in microfluidics
Generally, Janus particles in microfluidics are synthesized by solidifying Janus droplets using phase separation, polymerization, or other processes. Using a planar sheath-flowing system, monodisperse Janus droplets comprising two immiscible organic phases (polymerizable phase and non-polymerizable phase) are generated in a co-flowing aqueous phase. Subsequent off-chip polymerization produces monodisperse polymer particles with anisotropic shapes.19 In order to create Janus particles using a sheath flow system, a Y-shaped channel is used to form a two-phase organic stream that enters a co-flowing aqueous stream to create a Janus droplet (Fig. 2a). This setup has been demonstrated with pigments of carbon black and titanium oxide dispersed in an acrylic monomer to prepare separate black and white monomers. After the droplet formation and off-chip thermal polymerization, bicolored Janus particles with electrical anisotropies are obtained (Fig. 2b).20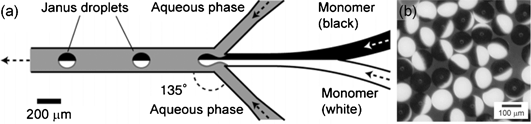 | ||
| Fig. 2 (a) Channel and flow configuration to generate the bicolored Janus droplets in a planar microfluidic geometry. (b) Synthesized bicoloured Janus particles. Image reproduced from ref. 20 with permission from Wiley publishing. | ||
Janus particles with a sharp interface between the phases can also be achieved by continuous microfluidic synthesis. As shown in Fig. 3a, two liquid monomers (M1 and M2) (mixed with photoinitiator) are introduced into two central channels, with side channels containing sodium dodecylsulfate (SDS) aqueous solution acting as sheath flows. Janus droplets are produced and released at the exit of the central channels, and since M1 and M2 are immiscible, there is a sharp interface within the Janus droplet composed between M1 and M2. In the following rapid polymerization process, the sharp interface is retained in the prepared Janus particles (Fig. 3b–d). Using the same device with varying flow conditions in each channel, ternary-structured Janus particles can be prepared (Fig. 3e). The biggest advantage of this technique is that the phase ratio between M1 and M2 in the Janus particles can be conveniently adjusted by varying the flow rate of M1 and M2 with the help of the fast photo-polymerization process. Selective functionalization of the Janus particles, rendered possible by the different chemical composition of M1 and M2, may further impart the particles with tailored properties for different applications.21
 | ||
| Fig. 3 (a) Schematic of formation of droplets with ternary structures. Monomers M1 and M2 are injected in intermediate and central channels, respectively. (b, c, d) Optical microscopy images of Janus particles. Bright and dark phases are polymers of M1 and M2, respectively. (e) Janus particles with ternary structures. Image reproduced from ref. 21 with permission from ACS publishing. | ||
The properties of Janus particles are usually determined by their composition and structure. Because of this, structural complexities are a prerequisite to build multifunctional Janus particles. In this regard, Weitz and colleagues developed a new technique to realize Janus particles from two immiscible fluids.22 By intentionally tuning the wettability of the inner surface of the microfluidic channel (i.e., from hydrophilic to hydrophobic), oil–water–oil double emulsion droplets from immiscible monomer mixtures can be formed. The photopolymerization used to “freeze” the asymmetric droplet structure transforms the droplets into Janus particles with a hydrophilic–organophilic structure. Janus particles can also be formed with one side composed of hydrogel and the other aggregated colloidal particles by inducing phase separation through a thermal treatment (Fig. 4).23 In this approach, the internal architecture of the Janus particles can be finely-tuned, making it more versatile for a variety of applications. Magnetic fields can be further used to assist the phase separation between iron oxide nanoparticles (< 50 nm) and silica spheres (330 nm) dispersed in photocurable ethoxylated trimethylolpropane triacrylate (ETPTA).24 The results after polymerization are Janus particles with a net magnetic moment whose surfaces are decorated with silica particle arrays. By applying a rotating magnetic field, rotational and translational motion of the magnetic Janus particles can be realized.24
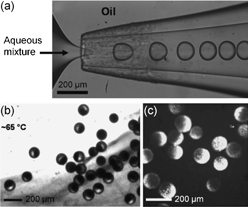 | ||
| Fig. 4 (a) Formation of droplets in a microfluidic device with a flow focusing geometry. (b, c) Janus particles with one side composed of aggregated colloidal particles. Image reproduced from ref. 23 with permission from Wiley publishing. | ||
All of the aforementioned Janus particles have dimensions at the micrometer scale (∼50 μm). In some cases, smaller Janus particles (nanometre sized) are highly desirable. Biphasic electrified jetting using side-by-side capillaries has proven to be a robust way to prepare Janus particles with submicrometer size.25 PEO mixed with sodium polystyrene sulphonate (PSS) and PEO with polyethylene imine (PEI) have been used in the two capillaries (or as jetting liquid) with an external electric potential ranging from 5 to 15 kV applied between the tip of the liquid and the counter-electrode (collecting the Janus particles). After the electrified jetting process, PEO Janus particles with one side doped by PEI and the other side incorporating PSS are prepared; they are roughly several hundred nanometers in size. Additionally, by modifying the chemical input of the two sides, green and red fluorescent emission from a single Janus particle is realized. Reduction of size to the submicron range opens up new possibilities for application, although the size distribution of these Janus nanoparticles may need to be narrowed if size uniformity is important, such as the case for in vivo or intracellular delivery of therapeutics.
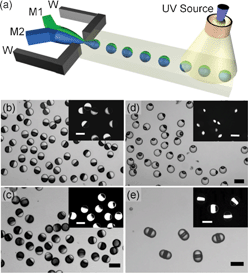
In addition to size limitations, most of the techniques discussed here can only prepare spherical Janus particles. In some cases, non-spherical Janus particles are needed. For example, endocytosis of particles is not only dependent on size, but also on shape. For instance, a rod-like particle may be advantageous for intracellular delivery.26 The in vivo transport and distribution of particles is also a sensitive function of shape, where a wormlike particle persists much longer in systemic circulation than its spherical conunterpart.27 It is worth mentioning that most of the Janus particles synthesized in microfluidics aforementioned are carried out in a two-phase system, limiting the throughput, shape, and functionality of the Janus particles. In this regard, Doyle and colleagues have developed a single-phase method which combines the merits of microscopic projection photolithography and microfluidics to continuously generate morphologically complex particles with multifunctionalities down to the colloidal length scale (Fig. 5).28–30 In a typical experiment, an acrylate oligomer stream containing a photosensitive initiator is passed through a polydimethylsiloxane (PDMS) microfluidic channel. A controllable UV light source irradiates the continuous flow through a mask, giving rise to the formation of particle arrays with shapes dictated by those of the mask. The rapid polymerization kinetics (< 0.1 s) and the oxygen-aided prohibition of the polymerization process near the PDMS channel wall ensures that the particles are formed in the stream and not at the wall. Such “flow-based” soft lithography enjoys the same fidelity as conventional lithographical techniques, and hence can be used to easily tune the shape of the Janus particles. By photopolymerizing the two streams containing PED-DA and PED-DA labelled with rhodamine coflowing in the PDMS channel, rectangular-shaped bifunctional Janus particles were synthesized. Nearly 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 particles per hour can be prepared using this technique.28
000 particles per hour can be prepared using this technique.28
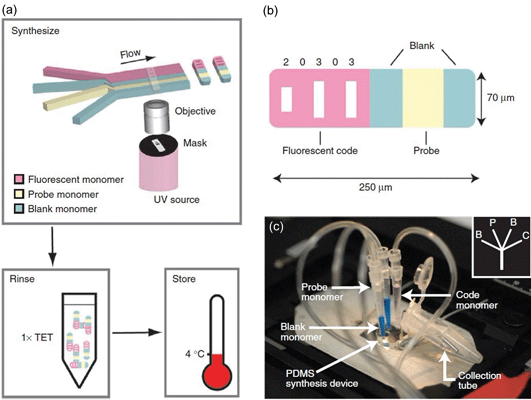 | ||
| Fig. 5 (a) Schematic diagram of the synthesis of single-probe particles. (b) A bar-coded particle with code 20303. (c) A photograph of the device mounted on the microscope for protein detections. Image reproduced from ref. 30 with permission from Nature publishing. | ||
Biomedical applications of Janus particles
The potential application of Janus particles in different fields is only emerging, and is probably limited only by our imagination. As a multifunctional nanomaterial, the intrinsic appeal of Janus particles is their tunable and controllable asymmetric structure, which allows for spatially controlled functionalities down to the nanoscale. This is particularly attractive for biomedical applications because of the synergistic potential for multiplexing, multi-level targeting, and combination therapies. A few examples below highlight these possibilities.In diagnostics, Janus disks composed of a fluorescent, graphically encoded region and a probe-loaded region can be used to sensitively detect DNA oligomers.29 In this case, acrylate-modified oligonucleotide probes are designed to detect specific DNA sequences. High-throughput analysis is achieved by scanning the Janus disks equipped with multiple probes in a flow-through device.29 The sensitivity of this technology is such that DNA oligomers can be easily detected without biotin-avidin-aided signal amplification at 500 attomolar. This concept has also been used in the capture and labelling of protein targets and the rapid microfluidic scanning of disk Janus particles for multiplexed detection.30 Using this concept, magnetic microparticles are incorporated into the Janus disks to achieve a DNA hybridization assay with multiplexing capabilities.31 The Janus disks can be rotated by an external magnetic field, enabling solution exchange through immobilization, particle decoding through orientation control and reaction enhancement through active stirring.31 In another example, a stimulus such as ionic strength can be used to induce assembly (analogous to encapsulation) or disassembly (analogous to release) of Janus particles for diagnostics or controlled release applications.11,12
In drug and gene delivery, targeting is vital to spare healthy cells from damage and to enhance the bioavailability of the drug. Current controlled release particles use conjugated ligands on the surface of the particles for targeting. It may be advantageous to decouple the targeting function from the therapeutic function in the same particle. One example uses bi-segmented metallic nanorods to deliver exogenous genes.32 One half of the nanorod is used to immobilize a targeting ligand while the other half is used to immobilize the nucleic acid. In this manner, the ligand density can be easily controlled by adjusting the length of the targeting segment of the nanorods, and the type of ligand can also be readily exchanged. Unfortunately, the metals used in this system have toxicity concerns. A Janus particle composed of biocompatible polymers would eliminate this concern, while offering the same benefit of independently- and spatially-controlled ligand incorporation. Another intriguing possibility is multi-level targeting, which is difficult to achieve with the current drug delivery systems. To improve targeted delivery of chemotherapeutics to tumours, for instance, it would be beneficial to use ligand A to target the leaky tumour vasculature and ligand B to target the specific tumour cells once the drug-loaded nanoparticles reach the tumour tissue. In principle, this can be accomplished with a Janus particle offering spatially distinct ligand patterns. Increasingly, drug therapies are also seeing the benefit of combination therapies, where multiple therapeutic agents act on different molecular pathways to exert a synergistic effect. An example is the delivery of Plk1 siRNA and paclitaxel to promote synergistic tumour suppression.33 The co-delivery sensitizes the tumour cells to paclitaxel, thereby requiring a much lower dose of the toxic paclitaxel to achieve a comparable therapeutic effect. Encapsulating compounds of widely different physical characteristics (size, charge, lipophilicity) in the same particle is not trivial, and again, Janus particles are well poised to address such a challenge. Immunization also provides a good example of the benefits of combination therapy. The nature and magnitude of the immune response generated by a vaccine is dependent on the type of antigen. For example, it may be advantageous to deliver a protein antigen and a DNA antigen to the same antigen presenting cell in order to stimulate a more comprehensive immune response involving both the Th1 and Th2 mechanisms.34 One can envision the application of a Janus particle to achieve that goal, even with the additional possibility of co-delivering immunoadjuvants to the same immune cell for an optimal immune response.
Another exciting trend of drug and gene delivery is the implementation of theranostics. Incorporating therapeutic, targeting, and imaging modalities all in one particle should maximize the efficacy of a therapy. A recent example demonstrated the potential of a designed Janus particle, where one side of the particle is covered by a rough gold surface and the other side is polystyrene (Fig. 6).35 The polystyrene hemisphere can be selectively functionalized with ligands for targeting, whereas the gold film can be used for sensing or imaging via surface-enhanced Raman scattering (SERS). The Janus nanoparticles show effective breast cancer cell targeting when anti-HER-2 antibodies are anchored on the polystyrene hemisphere surface, and visualization of their interaction with cells is made possible via SERS detection of the Rhodamine 6G adsorbed on the rough gold surface.
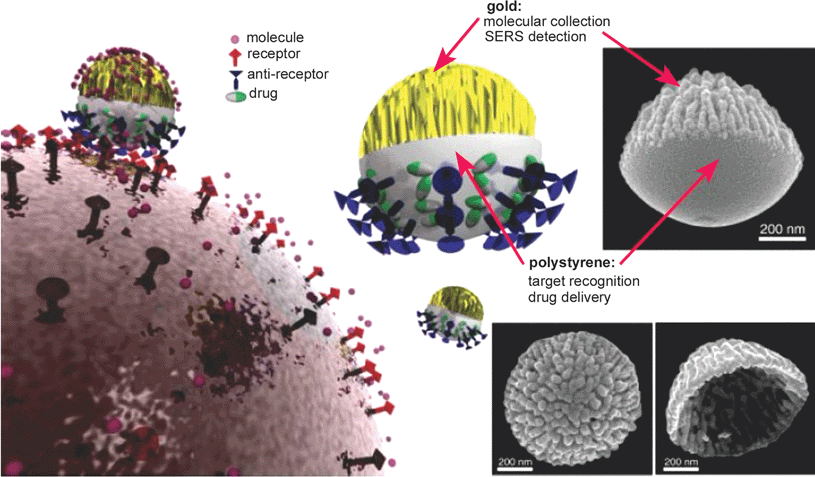 | ||
| Fig. 6 Janus particle composed of polystyrene surface and rough gold surface. It can be used as a nanoprobe for targeting, sensing, and drug delivery. Image reproduced from ref. 35 with permission from Wiley publishing. | ||
Finally, a recent report proposes the application of Janus particles for cell cultures. Cells and magnetic beads can be separately embedded in a hydrogel particle composed of alginate, making it possible to manipulate the cell via an external magnetic field with minimal contact between the cells and iron oxide particles.36
Conclusions
Microfluidics provides a unique platform to fabricate multifunctional Janus particles with more highly controlled structures and properties. Despite its promise, this field is still at an early stage. Microfluidics-based synthesis is fundamentally a bottom-up, self-assembly technique. Janus particles can also be made by top-down micro/nanofabrication techniques. Both bottom-up and top-down fabrication techniques have their pros and cons, and they are complementary to each other. Together, these two distinctly different approaches will be able to generate a wide range of Janus particles to fully achieve their potential. Robustness, versatility, and throughput are important qualifications for fabrication techniques. Top-down techniques traditionally tend to be more robust, although there is no reason why microfluidic-based fabrication cannot match the reproducibility if significant attention is paid to issues of flow parameters, chip design, and surface modification of channels. With its ability to precisely control and manipulate fluids in microenvironments, microfluidics probably would be the more versatile technique to create Janus particles with multiple domains. Throughput will depend on the type of Janus particles, and it is an issue that has to be addressed eventually. Ultimately, the following factors will dictate the choice of one fabrication technique over the other:(1) Structure: precise control of the phase separation of different components can lead to Janus particles with complex structures, although the laws of thermodynamics will ultimately limit the kind of structures that can be made. It remains to be seen how complex the structures will need to be, but even a binary structure could be quite useful. In the near-future, ternary structures should be able to satisfy the requirements of most biomedical applications, as discussed above for combination therapy or theranostic applications. Microfluidics appears to be well suited to fabricate these structures.
(2) Composition: the majority of Janus particles synthesized thus far are polymeric in nature. Metallic and inorganic Janus particles may also offer interesting opportunities because of their unique plasmonic, electronic, and magnetic properties. For example, targeted photothermal therapy may use a metallic Janus particle. If the synthesis required high temperature and harsh solvents, then additional effort would be required for a microfluidic approach. This is an under-researched area, but warrants more attention as the field moves forward.
(3) Size: most of the Janus particles fabricated by microfluidic approaches to date are large (> 50 μm), which would limit their potential biomedical applications. Particularly for in vivo applications, particles must be in the submicron range to navigate biological barriers efficiently, in fact the smaller the better. Generation of nanoscale Janus particles in microfluidics is still a significant challenge, and recent advances in nanofluidics may help address this challenge.
(4) Shape: non-spherical particles can exhibit unique properties that are not present in spherical ones. It is possible to synthesize nanoparticles of different shapes by self-assembly, with reported shapes including ellipsoidal, rod-like, donut-shaped, and fibrillar. However, control of these shapes is by and large empirical and it will not be easy to synthesize Janus particles with well-controlled shapes. In this regard, top-down fabrication techniques would be superior, although structural complexity would be limited.
In summary, there is ample room for innovation, particularly with respect to synthesizing Janus particles with sophisticated structure through microfluidic techniques. The field is only beginning to define the potential of these particles. Their applications in medicine are particularly exciting, especially for therapeutic delivery, where multi-functionality can maximize the efficacy of drug-, gene-, and immunotherapy.
Acknowledgements
This research was supported by National Institutes of Health (1DP2OD007209-01 and R01 HL-089764).References
- X. S. Fang, T. Y. Zhai, U. K. Gautam, L. Li, L. M. Wu, B. Yoshio and D. Golberg, Prog. Mater. Sci., 2011, 56, 175 CrossRef CAS.
- Y. Lei, S. K. Yang, M. H. Wu and G. Wilde, Chem. Soc. Rev., 2011, 40, 1247 RSC.
- S. K. Yang, F. Xu, S. Ostendorp, G. Wilde, H. Zhao and Y. Lei, Adv. Funct. Mater., 2011, 21, 2446 CrossRef CAS.
- Y. N. Xia, Y. J. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60 CrossRef CAS.
- J. X. Fang, B. J. Ding and H. Gleiter, Chem. Soc. Rev., 2011, 40, 5347 RSC.
- P. G. de Gennes, Rev. Mod. Phys., 1992, 64, 645 CrossRef.
- A. B. Pawar and I. Kretzschmar, Soft Matter., 2010, 31, 150 CAS.
- J. Du and R. K. O'Reilly, Chem. Soc. Rev., 2011, 40, 2402 RSC.
- T. Brugarolas, B. J. Park, M. H. Lee and D. Lee, Adv. Funct. Mater., 2011, 21, 3924 CrossRef CAS.
- M. Lattuada and T. A. Hatton, Nano Today, 2011, 6, 286 CrossRef CAS.
- Q. Chen, J. K. Whitmer, S. Jiang, S. C. Bae, E. Luijten and S. Granick, Science, 2011, 331, 199 CrossRef CAS.
- Q. Chen, S. C. Bae and S. Granick, Nature, 2011, 469, 381 CrossRef CAS.
- D. Dendukuri and P. S. Doyle, Adv. Mater., 2009, 21, 4071 CrossRef CAS.
- D. Hirsemann, S. Shylesh, R. A. De Souza, B. Diar-Bakerly, B. Biersack, D. N. Muller, M. Martin, R. Schobert and J. Breu, Angew. Chem., Int. Ed., 2012, 51, 1348 CrossRef CAS.
- G. Krylova, L. J. Giovanetti, F. G. Requejo, N. M. Dimitrijevic, A. Prakapenka and E. V. Shevchenko, J. Am. Chem. Soc., 2012, 134, 4384 CrossRef CAS.
- S. K. Yang, J. J. Xu, Z. Y. Wang, H. B. Zeng and Y. Lei, J. Mater. Chem., 2011, 21, 11930 RSC.
- J. He, M. T. Perez, P. Zhang, Y. Liu, T. Babu, J. Gong and Z. Nie, J. Am. Chem. Soc., 2012, 134, 3639 CrossRef CAS.
- J. I. Park, A. Saffari, S. Kumar, A. Guenther and E. Kumacheva, Annu. Rev. Mater. Res., 2010, 40, 415 CrossRef CAS.
- T. Nisisako and T. Torii, Adv. Mater., 2007, 19, 1489 CrossRef CAS.
- T. Nisisako, T. Torii, T. Takahashi and Y. Takizawa, Adv. Mater., 2006, 18, 1152 CrossRef CAS.
- Z. Nie, W. Li, M. Seo, S. Xu and E. Kumacheva, J. Am. Chem. Soc., 2006, 128, 9408 CrossRef CAS.
- C. -H. Chen, R. K. Shah, A. R. Abate and D. A. Weitz, Langmuir, 2009, 25, 4320 CrossRef CAS.
- R. K. Shah, J. -W. Kim and D. A. Weitz, Adv. Mater., 2009, 21, 1949 CrossRef CAS.
- S. -H. Kim, J. Y. Sim, J. -M. Lim and S. -M. Yang, Angew. Chem., Int. Ed., 2010, 49, 3786 CrossRef CAS.
- K. -H. Roh, D. C. Martin and J. Lahann, Nat. Mater., 2005, 4, 759 CrossRef CAS.
- S. E. A. Gratton, P. A. Ropp, P. D. Pohlhaus, J. C. Luft, V. J. Madden, M. E. Napier and J. M. DeSimone, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11613 CrossRef CAS.
- D. A. Christian, S. Cai, O. B. Garbuzenko, T. Harada, A. L. Zajac, T. Minko and D. E. Discher, Mol. Pharmaceutics, 2009, 6, 1343 CrossRef CAS.
- D. Dendukuri, D. C. Pregibon, J. Collins, T. A. Hatton and P. S. Doyle, Nat. Mater., 2006, 5, 365 CrossRef CAS.
- D. C. Pregibon, M. Toner and P. S. Doyle, Science, 2007, 315, 1393 CrossRef CAS.
- D. C. Appleyard, S. C. Chapin, R. L. Srinivas and P. S. Doyle, Nat. Protoc., 2011, 6, 1762 CrossRef.
- H. Lee, J. Kim, H. Kim, J. Kim and S. Kwon, Nat. Mater., 2010, 9, 745 CrossRef CAS.
- A. K. Salem, P. C. Searson and K. W. Leong, Nat. Mater., 2003, 2, 668 CrossRef CAS.
- T. M. Sun, J. Z. Du, Y. D. Yao, C. Q. Mao, S. Dou, S. Y. Huang, P. Z. Zhang, K. W. Leong, E. W. Song and J. Wang, ACS Nano, 2011, 5, 1483 CrossRef CAS.
- A. K. Salem, C. F. Huang, T. W. Kim, T. C. Wu, P. C. Searson and K. W. Leong, Nanotechnology, 2005, 16, 484 CrossRef CAS.
- L. Y. Wu, B. M. Ross, S. Hong and L. P. Lee, Small, 2010, 6, 503 CrossRef CAS.
- L. B. Zhao, L. Pan, K. Zhang, S. S. Guo, W. Liu, Y. Wang, Y. Chen, X. Z. Zhao and H. L. W. Chan, Lab Chip, 2009, 9, 2981 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
