Research highlights
Šeila
Selimović
ab and
Ali
Khademhosseini
*abcd
aCenter for Biomedical Engineering, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Cambridge, Massachusetts 02139, U. S. A. E-mail: alik@rics.bwh.harvard.edu
bHarvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, U. S. A.
cWyss Institute for Biologically Inspired Engineering, Harvard University, Boston, Massachusetts 02115, U. S. A.
dWorld Premier International – Advanced Institute for Materials Research (WPI-AIMR), Tohoku University, Sendai 980-8577, Japan
First published on 17th January 2012
Self-assembled curved microdevices
A major goal of tissue engineering is to generate 3D tissue constructs that faithfully represent their in vivo counterparts. To achieve this goal it may be possible to use microfluidic structures that enable the formation of biomimetic vascular structures. Such microfluidic devices often include a network of perfusion channels for delivery of nutrients to and removal of waste from cells.1–3 Despite a transformative potential, most of these structures are confined to two dimensions, which is an inadequate simplification of the living tissue.A paradigm shift in the manufacture of microfluidic devices has recently been introduced by Gracias and colleagues.4 They discovered that mechanically heterogeneous thin photoresist (SU-8) films curve upon conditioning with organic solvents and self-assemble into complex 3D structures. This vastly increases the usefulness of photoresist molds, beyond serving as planar masters in soft lithographic processes.
The authors exploited the formation of stress gradients in thin (∼10 μm) layers of crosslinked photoresist to enable bending of the molds. These stress gradients were formed because the photoresist molecules closer to the UV-source crosslinked more strongly than molecules on the far side of the film. Thus, when free standing photoresist films were solvated in acetone they developed larger stresses on the more strongly crosslinked side. The resulting stress differential was dependent on the magnitude of the UV-energy. Upon desolvation of the films in water or upon drying, the more strongly crosslinked side of the photoresist film underwent smaller compression compared to the weakly crosslinked side, resulting in warping of the material. This process was reversible, as after being soaked again in acetone, the photoresist films flattened and assumed their original shape. In addition, the reversibility of this process was tested to show that the hysteresis effect contributed to only a 12% difference in the radius of curvature after 100 cycles.
By selectively exposing different parts of a photoresist film using different UV-parameters (energy, position of the UV-source below or above the film), a range of complex 3D structures, some of them more than 1 cm in size, were generated. Among them, a cylindrical mesh, cubes with and without added patterns, a spiral, and a stretchy surface formed from a bidirectionally curved photoresist film stand out.
The stress differentials in materials can be used to curve objects in order to generate self-assembled structures and ultimately 3D microfluidic devices. To further utilize these structures for biological systems, biodegradable material may be used. Furthermore, this study demonstrates that dynamic material properties and architecture could be combined to generate structures that would be otherwise difficult to fabricate.
Immunomagnetic hooks for capturing circulating tumor cells
Methods for detecting circulating tumor cells (CTCs) in the blood have generated much interest as a diagnostic approach for analyzing the progression of cancer. An increase in the number of CTCs in the blood stream indicates an increase in tumor size or metastatic processes, while a decrease in CTC numbers usually signals recession of the cancer. Thus, accurate and precise counts of CTCs in a cancer patient's blood are often taken as indicators of the progression of the disease.5,6 Ultimately, CTC numbers also offer information on the efficacy of the cancer treatment to which patients are being subjected.Not surprisingly, recent advances in the field of microfluidics have been used to capture various cells types, such as CTCs. A noteworthy method of cell detection has recently been introduced by Zhang and colleagues, in which antibody-coated magnetic particles were utilized on a microchip for cell detection and capture.
In their work, Hoshino et al.7 used an immunoassay-based cell detection method due to its specificity to capture CTCs relative to morphological cell sorting approaches. To capture human colon cancer cells (COLO205), iron (II,III) oxide magnetic nanoparticles (100 nm in size) that were functionalized with EpCAM antibodies were used. In the study, these particles and cells were added to whole blood from patients. In this mixture, the immunolabelled magnetic particles selectively adhered to CTCs. The blood was then drawn into a fluidic chip, consisting of a wide fluidic channel. To generate the magnetic forces to capture the CTCs, the device was placed on a set of three permanent, rod-shaped magnets positioned with alternating orientations (Fig. 1).
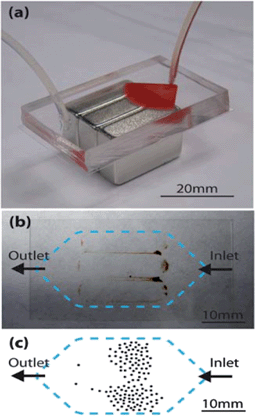 | ||
| Fig. 1 (a) Circulating tumor cell (CTC) detection chip placed on three magnets, with whole blood entering the chip through tubing on the right side. (b) Bottom collection slide with captured magnetic particles and cells. (c) Drawn example of the distribution of captured COLO205 cells. Figure adapted and reprinted with permission from the Royal Society of Chemistry from Hoshino et al.7 | ||
Due to the alternating N–S orientations of the magnets, a large magnetic field gradient could be generated. This resulted in high magnetic flux densities along the contact sides of the three magnets and allowed for capture of high numbers of cells. Additionally, the capture efficiency could be increased by placing the magnets parallel to the flow. Namely, in this case flowing particles and cells were exposed to the high flux density for a longer time than when the magnets were perpendicular to the blood flow. As expected, in the absence of the permanent magnets, no cells were captured.
At ferrofluid concentrations in the order of 10 μl ml−1 of whole blood and flow rates in the order of a few ml h−1, average capture rates reached 90%, and always exceeded 50%. Immunofluorescence analysis confirmed that the captured cells were indeed CTCs. Thus it appears that compared to commercially available systems, there is a 25% reduction in the concentration of the magnetic particles. Furthermore, most other CTC detection methods require relatively high CTC numbers for accurate detection (∼1000 ml−1). In contrast, the microchip-based immunomagnetic technique can detect CTCs at concentrations as low as 5 cells ml−1 and a CTC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) blood cell ratio of 1
blood cell ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 109 with a high specificity and high capture rate, but higher concentrations were equally successfully detected.
109 with a high specificity and high capture rate, but higher concentrations were equally successfully detected.
Aside from the high accuracy and precision of the device, this CTC detection method stands out due to its simplicity and portability. This positions the device for potential use in point-of-care applications. In this context, it would be interesting to add an imaging module, such as those present in cellular phones, as well as an image analysis application, which would allow an on-the-spot analysis of the captured cells.
Microfluidics for geological studies
The detection and extraction of oil has been an important factor in economic development world-wide, and has influenced the research focus in disciplines ranging from physics to geology. Often studies on oil extraction, such as analyzing the effects of rock porosity, sedimentation, and flow in pore networks require native rock samples or, alternatively, expensive and time-consuming simulations.8–10 Recently, however, the field of microfluidics has been applied to these studies, since random porous structures can be cost-efficiently engineered on a microscale, while allowing for fundamental research of the relevant flow processes. Two recent papers, by Neeves and colleagues and Hassanizadeh and colleagues, offer particularly interesting approaches for studying two-phase flows in microscale porous media. The first by mimicking native rock porous structures and the latter by designing homogeneous and heterogeneous structures with controlled porosity.The paper by Gunda et al.11 details a method for design and fabrication of a “Reservoir-on-a-chip” (ROC), where the term reservoir refers to natural geological structures (e.g., porous rocks) containing water, oil and gases. The process began with a core from reservoir rock, which was imaged using focused ion beam-scanning electron microscopy to gain information about its 3D microstructure (Fig. 2a). Next, a 2D representation of these pores was generated and etched in silicon. The resulting network of fluidic channels was sealed against a glass slide to form a closed microfluidic network. The average depth of the channels (throats) was ∼40 μm, and a total of 2000 pores and 6000 throats were included in the hydrophilic ROC.
 | ||
| Fig. 2 (a) Design and fabrication scheme of the Reservoir-on-a-chip (ROC). (b) Displacement of mineral oil (grey solution) with water (black) in homogeneous and heterogeneous networks, with either hydrophilic or hydrophobic walls. Figure adapted and reprinted with permission from the Royal Society of Chemistry from Gunda et al.11 (a) and Wu et al.12 (b). | ||
The authors conducted waterflooding experiments on the ROC, a method of oil extraction in which water is used to displace oil resident in rock structures. The ROC was first fully filled with a lubricant oil, similar to light paraffin oil, then the water phase was introduced at a constant flow rate of 100 μl min−1. The volume of oil collected at the device exit was compared to the original volume of oil stored in the ROC. The rate of oil recovery was observed to decrease with the amount of injected water, which was also indicated by the formation of stagnant oil phases (pockets of oil that could not be displaced by water at that flow rate). These pockets occurred predominantly in large pores as well as pores with the highest number of connecting throats. The maximum fraction of recovered oil was 65%. These observations correspond with those made in standard core-flooding studies. Furthermore, the application of the ROC allowed the researchers to observe a critical point in oil recovery, called the “breakthrough”, which is the time at which water starts exiting the porous structure (t = 13 min).
Currently, a minor drawback of the ROC is its structural weakness. For example, the current system prohibits the application of water flow rates used in the field (∼ft day−1 or 1000 μl min−1), because of potential device failure. A solution could be found in using tougher materials and strengthening the bonding between the pores and the glass slide. But such approaches may lead to better understanding and higher efficiency of the of the oil extraction process.
The work by Wu et al.12 differs in the method of generating the design of the pore-space. Here, an algorithm based on 2D Voronoi diagrams was used. Briefly, this approach is based on a spatial object and an associated Voronoi cell, which is a set of all points that are the same distance away from this and other objects in the space. This algorithm was applied to generate large homogeneous porous structures like the ones shown in Fig. 2b. Heterogeneous geometries were created by removing 10% of the objects, which led to the formation of large pores. This was done both randomly and with a set spatial probability. The resulting patterns were used to generate PDMS-based microfluidic devices. The devices were considered water-wetting for a brief period after exposure to the plasma; after this recovery time the device channels became hydrophobic.
The researchers measured water permeability in the porous structure (at pressures in the order of 10–100 kPa) and compared it with simulations. Both approaches yielded similar permeability values, in the order of 0.4–0.6 μm2, depending on the porosity. Heterogeneous networks had larger porosities and therefore larger permeabilities. Furthermore, in water flooding experiments homogeneous networks showed no evidence of stagnant oil phases, regardless of the wetting properties of PDMS. However, in heterogeneous structures oil could be trapped, but the breakthrough occurred much earlier in hydrophobic than in hydrophilic devices.
An advantage of Voronoi algorithmic approach is that both the porosity and the pore size can be controlled, which allows for more precise studies of water flow and permeability in porous structures. However, it is unclear how well the generated porous media represent native rock structures, and if so, which type of rock formation they mimic best.
Nonetheless, this avenue of research—Reservoir-on-a-chip and Rock-on-a-chip—is exciting, not only because it enriches the field of geology and other related disciplines, but also because the application of microfluidics to new problems can lead to innovative solutions in addressing the world's energy challenges.
References
- E. W. Young and D. J. Beebe, Fundamentals of microfluidic cell culture in controlled microenvironments, Chem. Soc. Rev., 2010, 39(3), 1036–1048 RSC.
- J. H. Yeon and J.-K. Park, Microfluidic cell culture systems for cellular analysis, Biochip Journal, 2007, 1(1), 17–27 Search PubMed.
- N. K. Inamdar and J. T. Borenstein, Microfluidic cell culture models for tissue engineering, Curr. Opin. Biotechnol., 2011, 22(5), 1–9 CrossRef.
- M. Jamal, A. M. Zarafshar and D. H. Gracias, Differentially photo-crosslinked polymers enable self-assembling microfluidics, Nat. Commun., 2011, 2, 527 CrossRef.
- J.-Y. Pierga, et al., Circulating Tumor Cell Detection Predicts Early Metastatic Relapse After Neoadjuvant Chemotherapy in Large Operable and Locally Advanced Breast Cancer in a Phase II Randomized Trial, Clin. Cancer Res., 2008, 14(21), 7004–7010 CrossRef CAS.
- P. Paterlini-Brechot and N. L. Benali, Circulating tumor cells (CTC) detection: Clinical impact and future directions, Cancer Lett., 2007, 253(2), 180–204 CrossRef CAS.
- K. Hoshino, et al., Microchip-based immunomagnetic detection of circulating tumor cells, Lab Chip, 2011, 11, 3449–3457 RSC.
- X. Zhao, M. J. Blunt and J. Yao, Pore-scale modeling: Effects of wettability on waterflood oil recovery, J. Pet. Sci. Eng., 2010, 71, 169–178 CrossRef CAS.
- S. Z. Rouhani and M. S. Sohal, Two-phase flow patterns: A review of research results, Prog. Nucl. Energy, 1983, 11(3), 219–259 CrossRef CAS.
- J. J. Rathmell, P. H. Braun and T. K. Perkins, Reservoir Waterflood Residual Oil Saturation From Laboratory Tests, JPT, J. Pet. Technol., 1973, 25(2), 175–185 CAS.
- N. S. K. Gunda, et al., Reservoir-on-a-Chip (ROC): A new paradigm in reservoir engineering, Lab Chip, 2011, 11, 3785–3792 RSC.
- M. Wu, et al., Single- and two-phase flow in microfluidic porous media analogs based on Voronoi tessellation, Lab Chip, 2012, 12, 253–261 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
