Rapid prototyping of three-dimensional microfluidic mixers in glass by femtosecond laser direct writing†
Yang
Liao
a,
Jiangxin
Song
a,
En
Li
b,
Yong
Luo
*c,
Yinglong
Shen
a,
Danping
Chen
a,
Ya
Cheng
*a,
Zhizhan
Xu
*a,
Koji
Sugioka
d and
Katsumi
Midorikawa
d
aState Key Laboratory of High Field Laser Physics, Shanghai Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, Shanghai, 201800, P. R. China. E-mail: ya.cheng@siom.ac.cn; zzxu@mail.shcnc.ac.cn; Fax: +86 6991 8021; Tel: +86 6991 8546
bCollege of Physics Science, Nankai University, Tianjin, 300071, P. R. China
cSchool of Pharmaceutical Science and Technology, Dalian University of Technology, Dalian, 116024, P. R. China. E-mail: yluo@dlut.edu.cn
dLaser Technology Laboratory, RIKEN - Advanced Science Institute, Hirosawa 2-1, Wako, Saitama, 351-0198, Japan
First published on 9th January 2012
Abstract
The creation of complex three-dimensional (3D) microfluidic systems has attracted significant attention from both scientific and applied research communities. However, it is still a formidable challenge to build 3D microfluidic structures with arbitrary configurations using conventional planar lithographic fabrication methods. Here, we demonstrate rapid fabrication of high-aspect-ratio microfluidic channels with various 3D configurations in glass substrates by femtosecond laser direct writing. Based on this approach, we demonstrate a 3D passive microfluidic mixer and characterize its functionalities. This technology will enable rapid construction of complex 3D microfluidic devices for a wide array of lab-on-a-chip applications.
Introduction
Microfluidics is a rapidly emerging technology that enables miniaturization and integration for biological, chemical, and medical applications. By integrating fluidic functions such as valving, metering, mixing, transport, and separation on a single substrate, microfluidic systems can be used to control and manipulate tiny volumes of liquids with high precision, enabling downsizing of both chemical and biological analysis.1,2 In particular, the fluid mixing is an essential function required by most microfluidic systems. However, fast and efficient fluid mixing inside microchannels is usually difficult to achieve due to the laminar nature of microflows characterized by low Reynolds numbers. Recently, various passive mixers have been developed to achieve efficient mixing by utilizing three-dimensional (3D) geometric structures to induce disturbance in the fluids.3–11 Nevertheless, the fabrication of 3D microfluidic structures with arbitrary geometries is still challenging if not impossible, because today's mainstream microfluidic fabrication techniques heavily rely on the well-established 2D planar lithographic approach. Therefore, additional stacking and bonding procedures are often required in the construction of 3D microfluidic systems.As a direct and maskless fabrication technique, femtosecond laser micromachining provides a straightforward approach for high-precision spatial-selective modification inside transparent materials through nonlinear optical absorption.12 The unique ability of femtosecond laser direct writing to simultaneously alter the chemical and optical properties in bulk glass materials opens up new avenues for fabricating a variety of integrated microfluidic and optofluidic micro-systems/devices such as microfluidic lasers,13 nanoaquariums for observing living organisms,14,15 and optofluidic sensors with various functions including refractive index monitoring,16 single-cell detection and manipulation,17,18 and rapid screening of algae populations.19 Currently, there are mainly two strategies for fabricating 3D microfluidic channels embedded in glasses using a femtosecond laser. The first strategy employs femtosecond laser modification inside glass materials followed by chemical wet etching.20–22 Unfortunately, with this technique the length of the microfluidic channel that can be fabricated is usually limited to a few millimetres, due to the limited etch ratio between the areas with and without the femtosecond irradiation. Another strategy is to perform femtosecond laser 3D drilling from the rear surface of the glass in contact with distilled water, in which the water introduced into the microchannel can help to remove the ablated debris.23,24 However, when the length of the channel increases, the debris can no longer be ejected from the microchannel which in turn causes clogging and termination of the microchannel. So far, the microchannels fabricated by these methods often suffer from their insufficient lengths for practical microfluidic applications.
To address the above-mentioned issues, recently we have developed a new strategy to fabricate microchannels with nearly unlimited lengths and arbitrary geometries by femtosecond laser direct writing in mesoporous glass immersed in water followed by postannealing, realizing long square-wave-shaped microchannels25 and large-volume microfluidic chambers.26 In this article, we demonstrate rapid fabrication of complex 3D microchannel systems using this newly established technique. Particularly, a passive microfluidic mixer consisting of geometrically complex 3D microchannels is fabricated and characterized, showing superior mixing efficiency as compared to 1D microfluidic channels.
Experimental
Fabrication process
For fabrication of hollow 3D microchannels inside a glass chip, high-silica mesoporous glass was used as the substrate, which was produced by removing the borate phase from phase-separated alkali-borosilicate glass in a hot acid solution.27 The composition of the porous glass is approximately 95.5SiO2-4B2O3-0.5Na2O(wt.%). The pores with a mean diameter of ∼10 nm are distributed uniformly in the glass and occupy 40% of the glass volume. Particularly, these pores form a 3D connective network in the glass which allows liquid to flow through (see the ESI† for fabrication details).The 3D femtosecond laser micromachining system consists of a femtosecond laser source (Coherent, Inc., center wavelength: ∼800 nm, pulse width: ∼50 fs, repetition rate: 250 kHz), an imaging system, a computer-controlled XYZ translation stage, beam control devices and delivery optics, as shown in Fig. 1a. A long-working-distance water-immersed objective was employed for focusing the beam into the mesoporous glass sample, which was fixed in a petri dish filled with distilled water. The petri dish was placed on the translation stage.
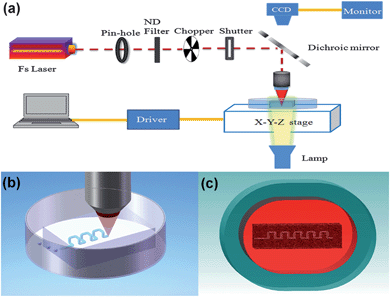 | ||
| Fig. 1 (a) Schematic diagram of 3D femtosecond laser machining system. Flow chart of fabrication procedures: (b) laser direct writing inside the mesoporous glass and (c) postannealing. | ||
The fabrication procedure consists of the following two steps as shown in Fig. 1b and 1c: (1) direct formation of 3D microchannels in the mesoporous glass immersed in water by femtosecond laser ablation; and (2) postannealing of the glass substrate at ∼1150 °C by which the mesoporous glass can be consolidated. After the consolidation process, all the nanopores in the porous glass collapse; however, the fabricated microchannels can survive due to their large diameter. Moreover, the porous glass substrate, which is opaque due to the scattering of the nanopores, becomes highly transparent after the consolidation process. Further details regarding the fabrication procedure and mechanisms are described elsewhere.25
Mixing experiments
To demonstrate the mixing efficiency of the 3D microfluidic mixer, fluid mixing experiments were carried out in 1D and 3D microfluidic channels by simultaneously injecting two fluorescent dye solutions (fluorescein sodium and Rhodamine B with a concentration of 1 mg ml−1 in water) into the flow channels at the same flow rate of 10 μl min−1 using syringe pumps, resulting in a flow rate of 8.8 cm s−1 and a Reynolds number of 5.3. The red and green fluorescences were imaged using a fluorescence microscope (Olympus, Inc. BX51) with two fluorescence filter sets, respectively. The mixing result was obtained by superimposing the two fluorescence images at a 50/50 ratio with image processing software (Media Cybernetics, Inc. Image-Pro Plus 6.0).Results and discussion
Helical microchannel and microfluidic channels with overpass structures
For the fabrication of microchannels by direct-writing in mesoporous glass immersed in water, the water mainly plays two roles: (1) dispersing the debris produced by laser ablation; and (2) producing bubbles to expel the debris from the channel through the outlet, which in turn promotes the water circulation. In this experiment, the high-repetition-rate femtosecond laser pulses with a pulse energy of ∼2 μJ were tightly focused by a water-immersed objective (N.A. = 1.0). Under such conditions, a peak laser intensity of ∼8 × 1015 W cm−2 can be reached in a tiny focal volume with a diameter of ∼0.8 μm, which is sufficiently strong for inducing nonlinear optical absorption and ablation of the glass material. The significant amount of heat and strong shock waves generated by the femtosecond laser ablation can facilitate water circulation and help to remove the debris from the outlet of the microchannel. Since the high-repetition-rate laser pulses could also lead to temporary evacuation of water in the laser action zone by semi-continuous irradiation, a chopper operated at 1 kHz with a duty of 50/50 was used (see Fig. 1a) to modulate the femtosecond laser pulse train during the ablation process, so that the water can always fill the ablation zone by infiltration through the mesoporous network.Fig. 2a shows a 3D helical microchannel with a total length of ∼1 cm and a diameter of ∼16 μm (i.e., corresponding to an aspect ratio of >600). The coil radius and the pitch of the helical channel are 100 μm and 50 μm, respectively. The homogeneous and debris-free helical channel was produced inside mesoporous glass by performing one slow scan (10 μm s−1) followed by three fast scans (100 μm s−1) over the whole microfluidic channel. The total fabrication time is less than 30 min (see the ESI† for more details and a movie of the direct writing process).
 | ||
| Fig. 2 (a) Optical micrograph of a 3D helical microchannel embedded in consolidated glass. Inset: fluorescence microscopy image of the microchannel filled with a solution of fluorescein. The confined fluorescent solution gives proof that the nanopores have all collapsed to form the consolidated substrate. (b) Optical micrograph of 3D microfluidic channels crossing over each other. Inset: fluorescence microscopy image of dye-filled microfluidic channels in (b). Red: Rhodamine B solution; green: fluorescein. | ||
The 3D nature of the femtosecond laser direct writing offers unique flexibility for constructing complex microfluidic networks. As an example, several spiral-like bends can be fabricated along a microfluidic channel to form the overpass structures so that one microchannel can cross over the others, as shown in Fig. 2b. The fluorescence micrograph presented in the inset of Fig. 2b indicates that indeed, the two microchannels do not intersect each other. In comparison with the “basketweave” structure fabricated by multilayer soft lithography,28 the overpass structure fabricated by femtosecond laser direct writing could lead to smoother transition and lower flow resistance.
3D microfluidic mixer
According to the chaotic theories, a passive micromixer based on the baker's transformation concept could provide ideal mixing performance.29 However, these types of mixers usually require complicated 3D geometries, thus they are difficult to fabricate by the conventional planar fabrication process. By use of the femtosecond laser direct writing, we fabricated a passive micromixer consisting of symmetrical 3D units as theoretically proposed by Ph. Carrière.30Fig. 3a and 3b present schematic illustrations of the designed 3D mixer which is composed of a Y-shape microchannel embedded 400 μm below the surface of the chip and a string of mixing units, connected to two opening inlets and one outlet. The inlets and outlet are all on the backside of the chip. Three circular slots surrounding the inlets and the outlet were also fabricated by femtosecond laser ablation, which were used as connection interfaces with plastic pipes of a diameter of 0.9 mm. | ||
| Fig. 3 Schematic diagrams of the 3D passive microfluidic mixer: (a) overview and (b) close-up view images. (c) Overview optical micrograph of the microfluidic mixer, (d) top view optical micrograph of two mixing units, and (e) cross sectional optical micrograph of the 3D microchannels. (f) Scanning electron micrograph of the inner wall of the fabricated microchannel. The dark sections in (e) are caused by the bubbles. | ||
As we have shown in previous work, the dimensions of the microchannel can be easily tuned by controlling the machining parameters, such as the numerical aperture of the objective, the laser pulse energy and the translation speed.26 In order to decrease the pressure drop of the micromixer, the width of the channel was set at 50 μm by decreasing the numerical aperture and meanwhile increasing the pulse energy (see the ESI† for fabrication details). Fig. 3c presents an overview of a 3D micromixer consisting of six mixing units. The length of all the horizontal and vertical channels in each mixing unit is 150 μm, as shown in Fig. 3d (top view) and 3e (side view). It can also be observed from Fig. 3e that the cross section of the channel is elliptical with a width of ∼50 μm and a depth of ∼75 μm. Fig. 3f shows the inner surface of the microchannel for evaluation of the surface roughness.
In order to demonstrate the 3D mixing mechanism based on the baker's transformation, numerical simulations were carried out by solving the microfluidic incompressible Navier–Stokes and convection diffusion equations using commercially available finite element analysis software (COMSOL Multiphysics 3.5) (see the ESI† for simulation details). Fig. 4a and 4b show the mixing results of two fluids in 1D and 3D microfluidic mixers, respectively. Clearly, in comparison with the 1D microfluidic channel, the 3D microfluidic mixer can provide far better mixing efficiency, which can be attributed to the repeated cutting and squeezing processes enabled by the alternatively arranged diverging and converging “T”-shaped microchannels.30
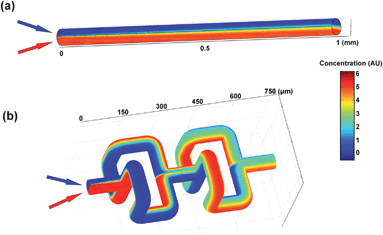 | ||
| Fig. 4 Numerical simulations of mixing results in (a) 1D and (b) 3D microfluidic mixers. Re = 5.3. | ||
Finally, Fig. 5a and 5b show the experimental results of mixing of the two fluorescent dye solutions (fluorescein sodium and Rhodamine B) in the 1D and 3D microfluidic mixers fabricated under the same direct writing conditions, respectively. Clearly, the mixing behaviors in these two structures agree well with the simulation results. For the 3D mixer, after passing through three mixing units (i.e., a length of 0.9 mm), the two fluids are well mixed, corresponding to a mixing time of about 10 ms. For comparison, Fig. 5a shows that in the 1D microfluidic channel, efficient mixing was not achieved after a propagation distance of ∼1300 μm.
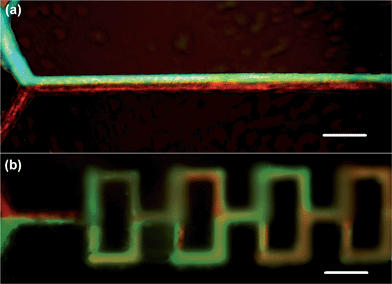 | ||
| Fig. 5 Fluorescence microscopy images of the (a) 1D and (b) 3D microfluidic mixing experiments, where two fluids (red and green) are mixed at Re = 5.3. Scale bars = 150 μm. | ||
Conclusion
In conclusion, we show that 3D microfluidic components with arbitrary geometries can be fabricated in glass substrates by femtosecond laser direct writing without using extra stacking and bonding procedures, making it possible for rapid prototyping of 3D microfluidic systems with enhanced efficiency, flexibility, fabrication precision, and cost-effectiveness. In particular, we demonstrate the fabrication of a 3D microfluidic mixer which shows superior mixing performance over its 1D counterpart. Our technique is promising for a broad spectrum of microfluidic applications based on compact and complex 3D microfluidic networks in the future.Acknowledgements
This work is supported by National Basic Research Program of China (2011CB808100) and National Natural Science Foundation of China (10974213, 60825406, 11174156, 61108015).Notes and references
- A. Manz, N. Graber and H. M. Widmer, Sens. Actuators, B, 1990, 1, 244 CrossRef.
- G. M. Whitesides, Nature, 2006, 442, 368 CrossRef CAS.
- N. T. Nguyen and Z. G. Wu, J. Micromech. Microeng., 2005, 15, R1 CrossRef.
- R. H. Liu, M. A. Stremler, K. V. Sharp, M. G. Olsen, J. G. Santiago, R. J. Adrian, H. Aref and D. J. Beebe, J. Microelectromech. Syst., 2000, 9, 190 CrossRef.
- A. D. Stroock, S. K. Dertinger, A. Ajdari, I. Mezic, H. A. Stone and G. M. Whitesides, Science, 2002, 295, 647 CrossRef CAS.
- D. Therriault, S. R. White and J. A. Lewis, Nat. Mater., 2003, 2, 265 CrossRef CAS.
- H. Chen and J. C. Meiners, Appl. Phys. Lett., 2004, 84, 2193 CrossRef CAS.
- F. Schönfeld, V. Hessel and C. Hofmann, Lab Chip, 2004, 4, 65 RSC.
- S. J. Park, J. K. Kim, J. Park, S. Chung, C. Chung and J. K. Chang, J. Micromech. Microeng., 2004, 14, 6 CrossRef.
- H. M. Xia, S. Y. M. Wan, C. Shu and Y. T. Chew, Lab Chip, 2005, 5, 748 RSC.
- T. Yasui, Y. Omoto, K. Osato, N. Kaji, N. Suzuki, T. Naito, M. Watanabe, Y. Okamoto, M. Tokeshi, E. Shamoto and Y. Baba, Lab Chip, 2011, 11, 3356 RSC.
- R. R. Gattass and E. Mazur, Nat. Photonics, 2008, 2, 219 CrossRef CAS.
- Y. Cheng, K. Sugioka and K. Midorikawa, Opt. Lett., 2004, 29, 2007 CrossRef.
- Y. Hanada, K. Sugioka, H. Kawano, I. S. Ishikawa, A. Miyawaki and K. Midorikawa, Biomed. Microdevices, 2008, 10, 403 CrossRef.
- Y. Hanada, K. Sugioka, I. S. Ishikawa, H. Kawano, A. Miyawaki and K. Midorikawa, Lab Chip, 2011, 11, 2109 RSC.
- A. Crespi, Y. Gu, B. Ngamsom, H. J. W. M. Hoekstra, C. Dongre, M. Pollnau, R. Ramponi, H. H. van den Vlekkert, P. Watts, G. Cerullo and Osellame, Lab Chip, 2010, 10, 1167 RSC.
- M. Kim, D. J. Hwang, H. Jeon, K. Hiromatsu and C. P. Grigoropoulos, Lab Chip, 2009, 9, 311 RSC.
- F. Bragheri, L. Ferrara, N. Bellini, K. C. Vishnubhatla, P. Minzioni, R. Ramponi, R. Osellame and I. Cristiani, J. Biophotonics, 2010, 3, 234 CrossRef CAS.
- A. Schaap, Y. Bellouard and T. Rohrlack, Biomed. Opt. Express, 2011, 2, 658 CrossRef.
- A. Marcinkevicius, S. Juodkazis, M. Watanabe, M. Miwa, S. Matsuo, H. Misawa and J. Nishii, Opt. Lett., 2001, 26, 277 CrossRef CAS.
- K. Sugioka, Y. Cheng and K. Midorikawa, Appl. Phys. A: Mater. Sci. Process., 2005, 81, 1 CrossRef CAS.
- K. Sugioka, Y. Hanada and K. Midorikawa, Laser Photonics Rev., 2010, 4, 386 CrossRef CAS.
- Y. Li, K. Itoh, W. Watanabe, K. Yamada, D. Kuroda, J. Nishii and Y. Y. Jiang, Opt. Lett., 2001, 26, 1912 CrossRef CAS.
- K. Ke, E. F. Hasselbrink and A. J. Hunt, Anal. Chem., 2005, 77, 5083 CrossRef CAS.
- Y. Liao, Y. Ju, L. Zhang, F. He, Q. Zhang, Y. Shen, D. Chen, Y. Cheng, Z. Xu, K. Sugioka and K. Midorikawa, Opt. Lett., 2010, 35, 3225 CrossRef.
- Y. Ju, Y. Liao, L. Zhang, Y. Shen, Q. Zhang, D. Chen, Y. Cheng, Z. Xu, K. Sugioka and K. Midorikawa, Microfluid. Nanofluid., 2011, 11, 111 CrossRef.
- D. Chen, H. Miyoshi, T. Akai and T. Yazawa, Appl. Phys. Lett., 2005, 86, 231908 CrossRef.
- J. R. Anderson, D. T. Chiu, R. J. Jackman, O. Cherniavskaya, J. C. McDonald, H. Wu, S. H. Whitesides and G. M. Whitesides, Anal. Chem., 2000, 72, 3158 CrossRef CAS.
- S. Wiggins and J. M. Ottino, Philos. Trans. R. Soc. London, Ser. A, 2004, 362, 937 CrossRef.
- P. Carrière, Phys. Fluids, 2007, 19, 118110 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the mesoporous glass preparation, the numerical simulations of mixing results, fabrication of 3D helical microchannel and 3D micromixer, movie of the direct writing process. See DOI: 10.1039/c2lc21015k |
| This journal is © The Royal Society of Chemistry 2012 |
