A microfluidic device for whole-animal drug screening using electrophysiological measures in the nematode C. elegans†‡
Shawn R.
Lockery
*a,
S. Elizabeth
Hulme
b,
William M.
Roberts
a,
Kristin J.
Robinson
a,
Anna
Laromaine
b,
Theodore H.
Lindsay
a,
George M.
Whitesides
b and
Janis C.
Weeks
a
aInstitute of Neuroscience, 1254 University of Oregon, Eugene, OR 97403-1254, USA. E-mail: shawn@uoregon.edu; Fax: 541-346-4548; Tel: 541-346-4590
bDepartment of Chemistry and Chemical Biology, Harvard University, 12 Oxford St., Cambridge, MA 02138, USA
First published on 26th April 2012
Abstract
This paper describes the fabrication and use of a microfluidic device for performing whole-animal chemical screens using non-invasive electrophysiological readouts of neuromuscular function in the nematode worm, C. elegans. The device consists of an array of microchannels to which electrodes are attached to form recording modules capable of detecting the electrical activity of the pharynx, a heart-like neuromuscular organ involved in feeding. The array is coupled to a tree-like arrangement of distribution channels that automatically delivers one nematode to each recording module. The same channels are then used to perfuse the recording modules with test solutions while recording the electropharyngeogram (EPG) from each worm with sufficient sensitivity to detect each pharyngeal contraction. The device accurately reported the acute effects of known anthelmintics (anti-nematode drugs) and also correctly distinguished a specific drug-resistant mutant strain of C. elegans from wild type. The approach described here is readily adaptable to parasitic species for the identification of novel anthelmintics. It is also applicable in toxicology and drug discovery programs for human metabolic and degenerative diseases for which C. elegans is used as a model.
Introduction
Whole-animal screens in small model organisms are gaining acceptance as a means to augment traditional screens based on cultured cells and single-celled organisms.1 The trend toward whole-animal screening is driven in part by advances in genome sequencing, which have revealed a high degree of genetic and biochemical conservation between humans and small, genetically tractable organisms such as the nematode Caenorhabditis elegans,2 the fruit fly Drosophila melanogaster,3–5 and the zebra fish Danio rerio.6 A second factor driving this trend is the integration of systems for computerized detection of chemically induced phenotypes with systems that automatically move and sort test organisms.7–11 As a result, whole-animal testing can now be done earlier in the drug development pathway than ever before, in some cases as early as the primary screening of compound libraries.1,9 This change is significant because compounds in whole-animal screens are tested at the level of functional multicellular units including synapses, muscles, and organs rather than isolated cells. Thus, promising compounds (leads) are more likely to succeed in subsequent phases of drug development.The success of whole-animal screens in small model organisms depends critically upon the objective parameters that form the basis of the screen. Such parameters, called readouts or end-points, have therefore been the targets of considerable ingenuity. Readouts fall into two broad categories: (1) assessment of the quantity or anatomical disposition of a fluorescent marker at the cellular12–14 or organismal15,16 level, and (2) quantification of stereotypic behaviors such as crawling,17,18 swimming,19,20 or egg laying.21
Each type of readout represents a unique compromise between cost and information content. The information content of fluorescent-marker readouts is generally low12 unless image analysis is used,16,22 but the latter usually requires using a high numerical aperture objective lens with short working distance and narrow field of view, making this method expensive to parallelize for whole-animal screens. Behavioral readouts, by contrast, can be obtained with inexpensive optics and thus are more easily parallelized. However, behavioral readouts typically provide less information about the target of drug action because a given behavioral phenotype can be generated by many different biological mechanisms (e.g., cessation of locomotion can result from mechanisms as divergent as ion-channel blockade and disruption of cellular respiration). This drawback can lead to a high rate of detection of compounds that ultimately prove unsuitable for use as drugs (false positives). Thus, there is a need for readouts that provide more direct information about the target of drug action, and are practical to parallelize.
Here we present a new whole-animal screening method that uses a non-invasive electrophysiological readout of neuromuscular function. The screen is based on monitoring the activity of the nematode throat (pharynx), a muscular pump that contracts rhythmically to draw liquid nutrients into the digestive tract during feeding. As in the vertebrate heart, the pattern of rhythmic contractions of the nematode pharynx is generated primarily by the pharyngeal muscles themselves, and each contraction is associated with an action potential, a large voltage transient that can be recorded by electrodes placed on the surface of the body.23 By analogy to an electrocardiogram, such a recording in nematodes is called an electropharyngeogram (EPG). In addition to registering action potentials in the pharyngeal muscles, the EPG also registers the activity of neurons that regulate the rate of pharyngeal pumping in much the same way as the autonomic nervous system regulates the vertebrate heart. Thus, the EPG can be used to investigate the effects of drugs that act on neurons as well as muscles.
Using soft-lithography, we constructed a device that permits simultaneous recording of EPGs from eight nematodes before, during, and after delivery of a test compound. To validate the utility of the device, we demonstrate here that it rapidly and reliably detects the acute effects of anthelmintics (anti-nematode drugs) and also correctly distinguishes a specific drug-resistant mutant strain of C. elegans from wild type. In addition to facilitating the search for new anthelmintics, the device is expected to be useful in two broad areas: toxicology and drug discovery programs, in which C. elegans serves as a model for human diseases including a variety of metabolic and degenerative disorders.3
Methodology
Conventional EPG recording method
In the conventional EPG recording method,23 the worm is immersed in a bath of physiological saline and sucked partway into a saline-filled glass pipette (Fig. 1A). Pharyngeal activity is induced by including in the saline the neuromodulator serotonin, which initiates feeding behavior in C. elegans.24 The close apposition between the worm's body and the mouth of the pipette generates an electrical resistance (Rseal) between the lumen of the pipette and the bath. As a result of this resistance, electrical currents generated by pharyngeal activity can be detected directly, or by means of the voltage difference (V) generated between the pipette and the bath.23 | ||
| Fig. 1 Methods for recording electropharyngeograms (EPGs) A. Diagram of the conventional method. A worm is submerged in a bath of physiological saline and the anterior end is sucked into a tight-fitting, saline-filled glass pipette. B−F. Design of the microfluidic device for recording EPGs. B. Top view of the overall device. Arrows indicate the direction of fluid flow. C. Top view of a single recording module. The inset shows the funnel-shaped entrance to the worm trap and position of the head of the worm in the trap under headfirst recording conditions. Color indicates feature height (black, 50 μm; gray, 10 μm). D. Three dimensional rendering of the recording module. E, F. Enhancement of signal-to-noise ratio (SNR) by positioning worm snugly in the worm trap. E. Micrograph of the recording module and the associated EPG recording with the syringe pump turned off. F. Same as E but with the syringe pump turned on, forcing the worm's head into the trap. Anterior is to the right and the recordings are shown on the same vertical scale in both panels. In E and F, arrowheads indicate the posterior margin of the pharynx. | ||
The conventional method is ill-suited to drug screens in two key respects. First, to attain this recording configuration, the operator must capture the worm by manual adjustment of a micromanipulator that holds the pipette (not shown); this form of capture would be difficult to automate and parallelize. Second, drugs are delivered via the bath solution, thus requiring macroscopic volumes of drug solutions.
Microfluidic EPG device
To adapt the EPG recording approach for drug screening, we constructed a device that automatically loads individual worms into eight recording modules for simultaneous EPG recording from multiple worms (Fig. 1B–F). The device is fabricated from a single layer of polydimethylsiloxane (PDMS), using standard methods of soft-lithography25,26 and bonded to a glass substrate after exposure to an air plasma. The array of recording modules is served by a bifurcating, tree-like arrangement of distribution channels. When a saline solution containing worms is injected into the inlet port of the device, the solution flows through all open channels, causing animals to segregate passively into separate recording modules until each is occupied by a worm.8Within each recording module, the worm is carried by the flowing fluid until it reaches a constriction (henceforth “trap”) into which only the worm's head or tail, which are narrower than the rest of the body, can fit. The trapped worm obstructs the worm channel, diverting most of the flow into the side-arm channels. This arrangement allows test solutions injected into the inlet port to continue to flow past the trapped worms while the EPG is recorded. Two waste reservoirs collect perfusate from the worm trap and side-arms, respectively. For long-term experiments (> 6 h), the capacity of the waste reservoirs can be increased by inserting tight-fitting glass tubes.
Electrical recordings are made by means of metal electrodes inserted into the worm electrode ports and the joint inlet/reference electrode port (Fig. 1B). Each of the worm electrodes is connected to the positive input of one of a bank of eight differential amplifiers (see Experimental Methods). The common reference electrode is connected to the negative inputs of all amplifier channels. The reference electrode is hollow to permit perfusion of test solutions during EPG recordings.
To optimize electrical recordings and solution delivery, it is important that the head or tail of the worm enters the trap rather than one of the side-arm openings. Three aspects of the design ensure that this happens. First, the dimensions of the side-arm channel openings (10 μm × 20 μm) are small compared to the diameter of the worm (70–90 μm). Second, dimensions of channels leading away from the recording module are adjusted such that the calculated hydrodynamic resistance to flow through the side arms to Reservoir 2 is 20 times the resistance to flow through the worm trap to Reservoir 1. Thus, as a worm enters a recording module, ∼ 95% of the flow is toward the worm trap rather than the side arms. Third, the funnel-shaped entrance to the worm trap aligns the worm so that its head or tail is driven into the trap (Fig. 1E, F). The design of the recording module thus ensures a snug fit of the worm in the trap, creating a sufficiently large electrical resistance (Rseal) to record the EPG with a high signal-to-noise ratio (SNR; Fig. 1F), without unduly compromising the flow of test solutions around the worm.
Worms were held in position throughout an experiment by positive pressure exerted by solutions perfused into the device via a syringe pump. Flow rate was adjusted so that approximately one third of the pharynx of a headfirst worm remained in the funnel-shaped region of the worm channel where it was exposed to perfused solutions. During tailfirst recordings, a similar length of the worm's body was lodged in the worm trap, and the head was completely exposed to the solution. The optimal flow rate depended on the relative size of the worm and the worm trap, with the effective range of flow rates for young adult C. elegans being approximately 5 to 50 μL min−1. Below this range, the SNR was too low (e.g., Fig. 1E); above this range, the worm was often forced through the trap. Under typical recording conditions, median SNR was 100 (n = 7), which was sufficient to observe detailed features of the EPG recordings. The frequency and duration of pharyngeal action potentials observed in the microfluidic device were similar to those reported for the conventional method under similar conditions,24,27 indicating that the pressure exerted by the flowing solution did not affect pharyngeal pumping.
Results and discussion
Parallel microfluidic electropharyngeograms
The waveforms of EPGs recorded using the microfluidic device were similar to those obtained using conventional EPG recording methods (Fig. 2A, B).23,24,27–30 The EPGs recorded from worms oriented tailfirst in the trap were similar to EPGs from headfirst worms except that the polarity was reversed.23 Referring to the canonical headfirst recording configuration (Fig. 2A, B), the most prominent features were a positive spike (E), caused by the rapid depolarizing phase of the pharyngeal muscle action potential that initiates contraction, and a negative spike (R) caused by the rapid repolarization of the pharyngeal muscle that terminates the contraction.23 Smaller downward peaks, which have been attributed to activity of neurons that modulate pharyngeal pumping,23 were often visible between E and R transients.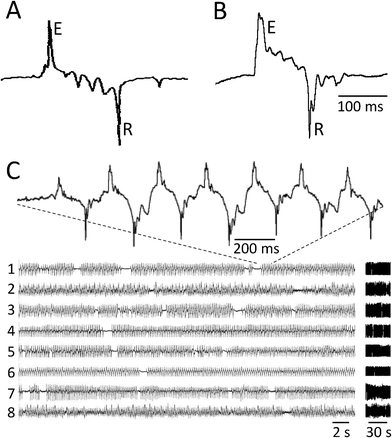 | ||
| Fig. 2 EPG recordings obtained by the conventional method and the microfluidic device. A. Pharyngeal action potential recorded using the conventional method shown in Fig. 1A (drawn to match Fig. 2A of reference24). E and R indicate the large positive (Excitatory) and negative (Relaxation) spikes, respectively, at the beginning and end of the action potential. The worm was oriented headfirst in the pipette. B. Pharyngeal action potential recorded using the microfluidic EPG device. Symbols and worm orientation are as in A. The scale bar in B refers to A and B. C. Simultaneous recordings from 8 worms in a single microfluidic EPG device. All worms except 2 and 8 were oriented headfirst in the worm trap. Vertical scales (voltage) were adjusted to produce similar signal amplitudes in all traces. The data on the left are duplicated at right on a compressed time axis, to illustrate how recordings are displayed in Figs 3 and 6. | ||
The array of recording modules made it possible to record from eight worms at one time (Fig. 2C). The probability that a recording module yielded a usable EPG recording was 0.75 (N = 348 modules). The most common cause of recording failure was an air bubble between the worm and one of the electrodes. In other cases, a worm failed to pump or there was more than one worm in the module. In standard saline (M9-5HT buffer, see Experimental Methods), pharyngeal pumping and EPG recordings continued for 6 to 8 h (Supplemental Fig. 1) and, although not investigated systematically, sometimes overnight. Thus, the device is suitable for medium-throughput experiments involving acute or semi-chronic exposure of multiple worms to small volumes of test compounds.
Delivery of test compounds
To demonstrate the ability to record EPGs in the presence of test compounds, we perfused the anthelmintic ivermectin during simultaneous EPG recordings from six worms (Fig. 3). Ivermectin inhibits pharyngeal muscles and extrapharyngeal neurons by activating a class of glutamate-gated chloride channels located in the plasma membrane.31,32 Perfusion of ivermectin blocked pharyngeal activity within 10–15 min,29,33 regardless of whether the worms were oriented headfirst or tailfirst. Pharyngeal activity was unaffected in worms that underwent a mock solution change (Fig. 3B), indicating that the cessation of activity was specific to the presence of the drug. Pharyngeal activity persisted in ivermectin-resistant mutants in which the three main muscular and neuronal targets of ivermectin are altered33 (Fig. 3C), indicating that the full effect of ivermectin in the EPG device requires the presence of previously established molecular targets. EPGs recorded in ivermectin also exhibited the expected reduction in the amplitude of the E and R spikes (Fig. 4).29,33 We conclude that test compounds reach the worms and have the expected pharmacological effects, for both headfirst and tailfirst orientation of the worm in the trap. | ||
| Fig. 3 Ivermectin effects on wild type and ivermectin-resistant worms. Each panel shows simultaneous EPG recordings from a single microfluidic EPG device, each containing both headfirst and tailfirst worms. Dashed lines replace data segments obscured by electrical artifacts during the solution change. Vertical scales (voltage) were adjusted to produce similar signal amplitudes in all traces; the horizontal scale bar (time) in C applies to all panels. In all experiments, worms were first perfused with control solution (M9-5HT buffer) for > 30 min before switching to drug or control solution containing 0.005% Fast Green as a visual marker. A. 3 μM ivermectin rapidly blocked EPG activity in wild type worms. B. EPG activity continued robustly during mock solution change in wild type worms. C. 3 μM ivermectin only partially inhibited EPG activity in ivermectin-resistant mutant worms (DA1316 avr-14(ad1302); avr-15(ad1051); glc-1(pk54)) compared to wild type worms (see A). | ||
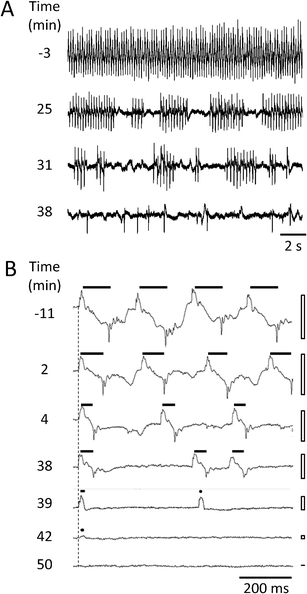 | ||
| Fig. 4 Changes in EPG waveform and frequency during ivermectin treatment. A and B each show representative recordings from a single wild type worm at the times indicated at left, relative to the switch from control solution to ivermectin (designated 0 min). Vertical gain (voltage) is the same for all traces within a panel. A. Changes in action potential timing and amplitude. This worm was tailfirst in the channel so E spikes are downward in the Figure. B. Changes in EPG waveform. This worm was headfirst in the channel and EPG traces are aligned (vertical dashed line) by the onset of a positive E spike, except for the bottom trace which occurred after EPG activity ended. The ivermectin concentration was 10 μM. Filled bars show E-to-R duration for each action potential waveform; filled circles indicate action potentials waveforms with an E but no R spike; open bars indicate voltage excursion of EPG waveforms in the adjoining trace. | ||
In addition, we detected two previously unreported effects of ivermectin. First, we observed an increase in the frequency of gaps in the otherwise regular pattern of action potential firing (Fig. 4A). In control solution, pumping was regular in frequency and large in amplitude, with approximately symmetric E and R spikes phases. In response to 0.1 μM ivermectin, however, the rhythm began to exhibit gaps that lengthened progressively over time, resulting in intermittent bouts of pumping. Pumping frequency within each bout decreased moderately over time, as did spike amplitude. Eventually only isolated pumps dominated by the E spike remained. Second, we observed a reduction in the duration of the action potential, measured as the interval between pairs of E and R spikes (Fig. 4B; see also Fig. 5C). The absence of such effects in worms undergoing a mock solution change (data not shown) indicates that these effects are a response to ivermectin, not a response to the device itself. Changes in action potential duration and the appearance of gaps have been reported in mutants with loss of function defects in the molecular target of the anthelmintic emodepside.27,34 This suggests that such effects may be common features of certain classes of anthelmintics.
 | ||
| Fig. 5 Effect of ivermectin on peak-to-peak EPG amplitude (A1), pump frequency (B1) and duration of pharyngeal action potentials (C1) for one representative wild type worm. The worm was first perfused with control solution (M9-5HT buffer for > 30 min) before switching to 0.1 μM ivermectin at t = 0. Each dot in A1, B1, C1 corresponds to a single pump as defined by the detection algorithm (see Experimental Methods). The events were divided into four epochs (−10 to −0.5 min, black; 0.3 to 20 min, magenta; 20 to 35 min, green; 35 to 50 min, blue). The corresponding probability distributions (PDFs) for the data points are shown in A2, B2, C2 using the same color scheme. | ||
Analysis of action potential waveform
Automated data analysis is an essential component of many practical screening approaches and such analysis methods have recently been introduced for EPG data.27,34 In addition to being objective and efficient, automated EPG analysis can measure parameters that provide valuable insights into the mode of action of a given test compound.34 To demonstrate application of automated methods to EPG recordings obtained in the microfluidic device, we quantified EPG records using three common metrics of pharyngeal activity: (1) peak-to-peak amplitude, defined as the voltage difference between the E and R peaks; (2) instantaneous pumping frequency, defined as the reciprocal of the time between successive E peaks; and (3) action potential duration, defined as the time interval between the E and R peaks in a single pumping event.Ivermectin caused a gradual reduction in all three measures (Fig. 5). The peak-to-peak amplitude began to decline toward zero ∼ 20 min after drug application, until pumps became undetectable after 50 min. The modal pumping frequency remained nearly constant at ∼ 5.2 Hz (B, black and magenta) until ∼ 20 min after drug application, when it too began to decline. Analysis of pumping frequency also revealed the appearance of many low frequency events as the drug began to take effect (Fig. 5B, 20–40 min). Inspection of the raw data showed that these events corresponded to the above-mentioned gaps in the regular firing pattern of action potentials (Fig. 4A). The duration of pharyngeal action potentials (Fig. 5C) began to decline ∼ 15 min after drug application from the initial modal duration of 104 ms (black and magenta) to 52 ms (green and blue). Neither the frequency nor duration of action potentials was noticeably altered during transient epochs of increased amplitude (x's in Fig. 5A1), which we speculate were caused by movement of the worm within its recording module.
Each of the effects shown in Fig. 5 is consistent with the activation of an inhibitory conductance in pharyngeal muscles, and is thus in agreement with the previously established mechanism of ivermectin action.32 This finding illustrates the ability of the present method to identity subtle drug effects that may provide valuable new information in mode-of-action studies.
Analysis of EPG magnitude
Automated analysis of EPG data is confounded by variations in action potential waveform across individual worms, and over time in response to test compounds28. This problem arises because extraction of spike frequency and related parameters requires the use of peak detection algorithms, which can be sensitive to the shape and amplitude of the underlying events. Although this difficulty can be mitigated to a large degree by customizing the peak detection algorithm for each worm, doing so on the large data sets generated by parallel EPG recordings may be impractical in some screening applications. Here we show that simply computing the magnitude of the EPG signal can be diagnostic for the effects of drugs and genetic backgrounds (Fig. 6). We computed EPG magnitude by taking the absolute value of the voltage at each time point and smoothing the result (see Experimental Methods). The background noise level, measured when pharyngeal pumping had stopped, was then subtracted, yielding a time series that we refer to as ||EPG||(t). This time series compactly represents two of the main features of each EPG recording; changes in the amplitude and frequency of action potentials over time. In Fig. 6A–C, ||EPG||(t) is plotted for three individual worms, one from each of the three conditions shown in Fig. 3. Group data (Fig. 6D,E) were obtained by normalizing individual ||EPG||(t) data to a value of 1 immediately prior to the solution change, then averaging across worms.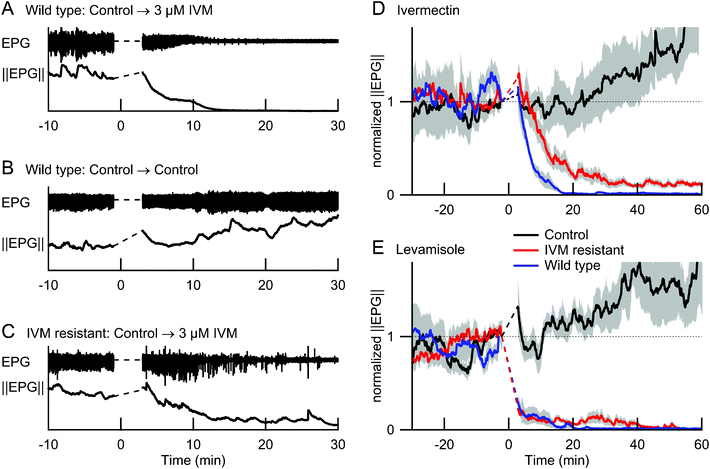 | ||
| Fig. 6 Effects of ivermectin and levamisole on the EPG and its magnitude ||EPG||(t) in wild type and ivermectin-resistant mutants. Dashed lines replace data segments obscured by electrical artifacts during the solution change. A–C show representative EPG data from three individual worms. D and E show ||EPG||(t), normalized to the value immediately before the solution change, and averaged across 5 or 6 worms per condition; grey shading shows ± 1 SEM. In all experiments, worms were first perfused with control solution (M9-5HT buffer) for > 30 min before switching at t = 0 to a test solution with 3 μM ivermectin (A,C and colored traces in D), 10 mM levamisole (colored traces in E), or control solution (B, black traces in D and E). Ivermectin-resistant worms were used for panel C and for the red traces in D and E; all other worms were wild type. | ||
The effects of ivermectin on wild type and ivermectin-resistant mutants were quantitatively different, even with small sample sizes (≤ 6 animals per group). Fig. 6D shows group data for the three conditions studied in the experiment of Fig. 3. By t = 6 min after exposure to ivermectin, the mean normalized ||EPG||(t) was significantly reduced in wild type worms compared to controls (p < 0.03; two-tailed t-test), but not in ivermectin-resistant mutants (p > 0.5). After 20 min exposure to ivermectin, ||EPG||(t) was significantly reduced in wild type and mutants compared to controls (p < 0.02), but remained significantly higher in mutants than in wild type worms for the duration of the experiments (t = 60 min; p < 0.02). The value of ||EPG||(t) in control animals increased over time; although further experiments are required, we speculate that worms were gradually pushed further into the traps as time progressed, increasing Rseal.
Fig. 6E shows a similar experiment in which the anthelmintic levamisole was used instead of ivermectin. As expected,35 levamisole caused rapid inhibition of pharyngeal pumping in both wild type and ivermectin-resistant worms compared to controls (p < 0.0001 at t = 6 min, for both comparisons). Significantly, the ion channels that levamisole targets are present on body wall muscles36 and are not required for normal pharyngeal pumping.37 Thus, the microfluidic EPG device can detect anthelmintic drug activity even when a drug may be acting at extrapharyngeal sites. We also found that, in contrast to the effects of ivermectin, the effects of levamisole on wild type and ivermectin-resistant mutants were indistinguishable. This finding indicates that the EPG device could be used to characterize the effects of drugs on a wide range of resistance mutants or other genetic backgrounds that could be useful in assigning test compounds to existing (or novel) modes of action.
Conclusions
The device presented here permits simultaneous, individualized recordings of electrical activity from up to eight nematodes during the application of drugs and other test compounds. The main advantages relative to the conventional EPG recording method are automation, reduced utilization of test compounds, and parallelization. The number of simultaneous recordings could be increased by recording from many devices in parallel, by increasing the number of recording modules per device, or both. The practical limit to the throughput of the microfluidic EPG approach is not yet known, but distribution channels for positioning more than 100 nematodes at a time have been demonstrated.8Modifications to the current design using existing technology could enhance its utility. For large numbers of simultaneous recordings it would be advantageous to add microfabricated electrodes38 to facilitate automated connection to the recording equipment. The quantity of test compound required could be reduced by incorporating on-chip drug reservoirs. Importantly, the dimensions of distribution channels and recording modules could be adjusted to accommodate other species of nematodes. This modification would allow for direct, medium-throughput assessment of compounds to treat nematodes that parasitize a wide range of hosts from plants to humans.39
Experimental methods
Nematodes
C. elegans strains were grown on Nematode Growth Medium (NGM) agar plates seeded with the OP50 strain of E. coli. All strains were obtained from the Caenorhabditis Genetics Center (CGC; Minneapolis, MN). Wild-type worms were Bristol N2 and Ivermectin (IVM) resistant mutants were DA1316 avr-14(ad1302); avr-15(ad1051); glc-1(pk54). Adult hermaphrodites were used for all experiments with the exception of SNR measurements, which also included some L4 animals. Animals were age-synchronized by one of two methods: (1) L4s were transferred to a seeded NGM plate and left overnight at room temperature to develop into adults; or (2) gravid adults were placed on seeded NGM plates to lay eggs for ∼ 6 h and then removed, with the plates maintained at room temperature until the adults matured.Device Fabrication
We fabricated devices using standard soft lithographic methods.26,27 Briefly, a silicon wafer master for the device was created by exposing a 55 μm layer of SU-8 2050 resist (Microchem, Newton, MA) through a transparency mask and developing the master in a bath of glycol monomethyl ether acetate (PGMEA). Masters were replica-molded in PDMS (Dow Corning Sylgard 184, Corning, NY) and treated with chlorotrimethylsilane (Aldrich, St. Louis, MO) to prevent adhesion of PDMS to the master. Holes for ports, inlets, and fluid reservoirs were formed using biopsy punches of the appropriate diameter (ports and inlets, 1.5 mm; reservoirs, 5 mm). PDMS castings were bonded to glass substrates after 30 s exposure to an oxidizing air plasma. After bonding, the capacity of each fluid reservoir was increased by inserting a 1.5 cm length of 5 mm glass tubing.Solutions
All experiments utilized M9 buffer40 containing KH2PO4, NaHPO4, NaCl and MgSO4, to which serotonin (5HT) was added to stimulate pharyngeal pumping.24 Stocks of serotonin creatine sulfate monohydrate (Sigma H7752; St. Louis, MO) were prepared in M9 buffer at 40 mM and held in small aliquots at −20 °C until use. Each day of an experiment, a fresh aliquot was thawed and diluted to 10 mM 5HT in M9 buffer. This “M9-5HT” buffer was the control medium to which drugs or other compounds were added. Stock solutions of ivermectin (10 mM; Sigma 8898) were made up in 100% dimethyl sulfoxide (DMSO; Fisher D-136; Fair Lawn, NJ) and held at −20 °C for no longer than 2 wk. Control experiments (not shown) indicated that DMSO at the final concentrations used for perfusions (≤ 0.1%) did not adversely affect EPG activity. Stock solutions of levamisole hydrochloride (100 mM; Sigma 31742) were made up in sterile dH20 and maintained at 4 °C. On the day of an experiment, IVM or LEV stocks were diluted to the final concentration in M9-5HT buffer that contained 0.005% Fast Green (Fisher F-99) as a visual indicator of drug flow within the device (see below); the dye-containing buffer was filtered (PALL Life Sciences 25 mm Acrodisc syringe filter with 0.2 μm HT Tuffryn Membrane; Port Washington, NY) before adding test compounds.Loading worms into the device
Young adult worms were transferred from growth plates into a glass well containing M9-5HT buffer and left to acclimate for 10 min. While viewed under transillumination on a Stemi SV6 binocular stereomicroscope (Carl Zeiss Inc., Thornwood, NY), 8 worms were picked using a loop tool and placed into the inlet port of the device, which had been preloaded with M9-5HT buffer. A 3 cc syringe filled with M9-5HT buffer connected to a length of 1.5 mm polyethylene tubing was then inserted into the inlet port and pressure was applied by gently pressing on the piston of the syringe to propel worms into the eight individual worm channels in the device. The tubing connected to the inlet port was then removed and the loaded device was moved to the EPG recording apparatus. The time required to load the device (1 min) was approximately the same as the time required to capture a single worm in a micropipette for a conventional EPG recording. Thus, the device yields an N-fold reduction in loading time, where N is the number of channels in the device.Electrophysiological recordings
EPGs were recorded via AC differential amplifiers (A−M Systems model 1700, Carlsborg, WA) connected to electrodes inserted into the device. Electrodes were made from 0.5 inch long passivated 17 gauge stainless steel tubes (0.058 inch OD, 0.0475 inch ID, New England Small Tube, Litchfield, NH).Recordings were made at voltage gains of 1000 × or 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × and filtered with a low-pass cutoff of 1 Hz and a high-pass cutoff of 1 kHz; recordings were further conditioned by a 60 Hz notch filter. Signals were displayed on oscilloscopes (TDS 2024B, Tektronix, Beaverton, OR) at a sweep speed sufficient to resolve the components of individual pharyngeal action potentials. Signals were recorded for later analysis using a data acquisition system (Micro1401-3, Cambridge Electronic Design (CED), Cambridge, UK) connected to a computer running Spike2 software (version 7.06a, CED). Data were sampled at 5 or 10 kHz per channel. An additional channel was used as a keystroke-controlled event marker (e.g., time of drug delivery). Data were typically acquired continuously in Spike 2, beginning when electrodes were inserted into the device and continuing until an experiment was terminated (generally 1–2 h after drug addition). For long recording sessions (e.g., overnight), Spike2 was set to acquire short data segments at regular intervals (e.g., 5 min of recording every 20 min).
000 × and filtered with a low-pass cutoff of 1 Hz and a high-pass cutoff of 1 kHz; recordings were further conditioned by a 60 Hz notch filter. Signals were displayed on oscilloscopes (TDS 2024B, Tektronix, Beaverton, OR) at a sweep speed sufficient to resolve the components of individual pharyngeal action potentials. Signals were recorded for later analysis using a data acquisition system (Micro1401-3, Cambridge Electronic Design (CED), Cambridge, UK) connected to a computer running Spike2 software (version 7.06a, CED). Data were sampled at 5 or 10 kHz per channel. An additional channel was used as a keystroke-controlled event marker (e.g., time of drug delivery). Data were typically acquired continuously in Spike 2, beginning when electrodes were inserted into the device and continuing until an experiment was terminated (generally 1–2 h after drug addition). For long recording sessions (e.g., overnight), Spike2 was set to acquire short data segments at regular intervals (e.g., 5 min of recording every 20 min).
Solution delivery
Solutions were delivered to EPG devices via a syringe pump (Harvard Apparatus PHD 2000; Holliston, MA) driving a pair of 3 cc syringes. One syringe was filled with M9-5HT buffer while the other contained M9-5HT buffer with 0.005% Fast Green to which was added nothing (for control experiments), drug, drug plus vehicle or vehicle alone. Each syringe was fitted with a 25 gauge stub needle that led via a 30 cm length of fine polyethylene tubing (BPE-T25, Instech Solomon, Plymouth Meeting, PA, USA) to the hollow metal reference electrode (see above). Following pilot experiments investigating the relationship between perfusion rate and EPG signal-to-noise (SNR) ratio (see Results), a perfusion rate of 6 μl min−1 was used. Solution changes were effected by removing the reference electrode connected to the first syringe from the fluid inlet port of the device and inserting the reference electrode connected to the second syringe. This procedure eliminated potential cross-contamination between solutions. The perfusion line from the syringe not in use was led to a waste receptacle. The latency between insertion of the electrode and arrival of the new solution at the worm's location, as visualized by dye movement, was approximately 60 s. The electrical artifact from switching reference electrodes was subsequently blanked from recordings shown in Figures.Calculation of ||EPG||(t)
A simple analysis, which did not involve identifying the occurrences of individual pharyngeal contractions, was used to quantify EPG magnitude over time. For this analysis EPG(t) was band-pass filtered in Spike2 (2–200 Hz, 2-pole Bessel filters), down-sampled to 500 Hz, full-wave rectified, and smoothed (exponential filter, time constant = 60 s), to yield ||EPG||(t), a measure that is proportional to both the frequency and strength of pharyngeal contractions. In experiments during which pharyngeal pumping ceased completely for a period of time, the baseline noise was removed by subtracting the minimum value of ||EPG||(t) during the experiment. The mean baseline noise determined in this way was 3.7 ± 0.5 μV (mean ± S.D., n = 24 worms). In experiments in which pharyngeal pumping never ceased, the baseline noise was assumed to be 3.7 μV.Analysis of EPG features
To analyze the effects of test solutions on the amplitude, frequency, and duration of pharyngeal action potentials (Fig. 5), we identified the onset of pharyngeal muscle contraction and relaxation during each pump cycle (Fig. 2). For this analysis, we inverted the sign of EPGs obtained from worms that were oriented tailfirst in the recording chamber. EPGs were high-pass filtered to aid in separating the E and R peaks from the slower components of the EPG waveform and from low-frequency noise. All positive and negative peaks that exceeded threshold values were then located, and event times measured as the times of the corresponding local maxima or minima in the unfiltered EPG. Positive and negative thresholds and high-pass filter frequencies were set by the user for each worm. Valid EPG events were defined as a suprathreshold positive peak followed by a suprathreshold negative peak with no intervening suprathreshold positive or negative peaks. For each valid EPG event we recorded the time interval between the positive and negative peaks, which corresponds to the duration of the contraction of the pharyngeal muscles,24 and the voltage difference between the positive and negative peaks (peak-to-peak amplitude), which reflects the size and speed of the muscle depolarization and repolarization. The peak-to-peak amplitude is also sensitive to changes in Rseal. We measured pump frequency as the reciprocal of the time interval between the positive peaks of successive valid EPG events.Calculation of SNR
Signal to noise ratio was defined as , where
, where  was the total variance of the EPG recording during vigorous pumping (∼ 5 Hz) and
was the total variance of the EPG recording during vigorous pumping (∼ 5 Hz) and  was the variance during periods in which pumping spontaneously ceased.
was the variance during periods in which pumping spontaneously ceased.
Measurement of Rseal
We determined typical values for Rseal by applying a voltage step between the positive electrode of a recording module and the common reference electrode of a device filled with M9 buffer (see above), and measuring the resulting electric current. Because of the small cross-sectional area of the channels in the microfluidic device, the electrical resistance was relatively high even when the recording channel was unoccupied by a worm (∼ 5 MΩ). This resistance increased to ∼ 6 MΩ with a worm loosely positioned in the channel, and typically increased by ∼ 200 kΩ with the worm snugly in the trap, from which we estimate that Rseal ≈ 200 kΩ, similar to seal resistances obtained in “loose-seal” patch recordings.41 Given the relatively small value of Rseal compared to the resistance of the unoccupied channel, it is perhaps surprising that the SNR increased 9-fold when the worm's head entered the trap, but this result is consistent with the conclusion that EPG is primarily the result of current flowing out of the mouth,24 which becomes electrically isolated from the rest of the body when the head is held snugly in the trap. It is unclear why the SNR also increased greatly when the tail of the worm entered the trap, but we speculate that the major return current pathway for the EPG may be through the worm's anus.Acknowledgements
Support: SRL, John Simon Guggenheim Foundation; NIH Challenge Grant RC1AI087059; and University of Oregon Technology Entrepreneurship Program. SEH, American Society for Engineering Education National Defense Science and Engineering Graduate Fellowship; Vertex Scholar Award. GMW, Bill and Melinda Gates Foundation award 51 308. GMW: Department of Energy DE-FG02-00ER4585. JCW, The Grass Foundation. Consultation: L. Avery, J. Dent, T. Geary, X. Liu, A. Sluder, J. C. W. Roberts.References
- J. Giacomotto and L. Segalat, Br. J. Pharmacol., 2010, 160, 204–216 CrossRef CAS.
- T. Kaletta and M. O. Hengartner, Nat. Rev. Drug Discovery, 2006, 5, 387–398 CrossRef CAS.
- E. Bier, Nat. Rev. Genet., 2005, 6, 9–23 CrossRef CAS.
- R. Drysdale, Methods Mol. Biol., 2008, 420, 45–59 CAS.
- G. M. Rubin and E. B. Lewis, Science, 2000, 287, 2216–2218 CrossRef CAS.
- G. Kari, U. Rodeck and A. P. Dicker, Clin. Pharmacol. Ther., 2007, 82, 70–80 CrossRef CAS.
- S. E. Hulme, S. S. Shevkoplyas, A. P. McGuigan, J. Apfeld, W. Fontana and G. M. Whitesides, Lab Chip, 2010, 10, 589–597 RSC.
- S. E. Hulme, S. S. Shevkoplyas, J. Apfeld, W. Fontana and G. M. Whitesides, Lab Chip, 2007, 7, 1515–1523 RSC.
- M. F. Yanik, C. B. Rohde and C. Pardo-Martin, Annu. Rev. Biomed. Eng., 2011, 13, 185–217 CrossRef CAS.
- D. Wlodkowic, K. Khoshmanesh, J. Akagi, D. E. Williams and J. M. Cooper, Cytometry A, 2011, 79, 799–813 CrossRef.
- R. T. Peterson, R. Nass, W. A. Boyd, J. H. Freedman, K. Dong and T. Narahashi, NeuroToxicology, 2008, 29, 546–555 CrossRef CAS.
- R. Pulak, in Methods in Molecular Biology, ed. K. Strange, 2006, 351, pp. 275–286. Search PubMed.
- W. A. Boyd, M. V. Smith, G. E. Kissling and J. H. Freedman, Neurotoxicol. Teratol., 32, 68–73 CrossRef CAS.
- S. J. Gosai, J. H. Kwak, C. J. Luke, O. S. Long, D. E. King, K. J. Kovatch, P. A. Johnston, T. Y. Shun, J. S. Lazo, D. H. Perlmutter, G. A. Silverman and S. C. Pak, PLoS One, 5, e15460 Search PubMed.
- C. B. Rohde, F. Zeng, R. Gonzalez-Rubio, M. Angel and M. F. Yanik, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 13891–13895 CrossRef CAS.
- M. M. Crane, K. Chung and H. Lu, Lab Chip, 2009, 9, 38–40 RSC.
- S. D. Buckingham and D. B. Sattelle, Invertebr. Neurosci., 2008, 8, 121–131 CrossRef.
- J. A. Carr, A. Parashar, R. Gibson, A. P. Robertson, R. J. Martin and S. Pandey, Lab Chip, 2011, 11, 2385–2396 RSC.
- K. Chung, M. Zhan, J. Srinivasan, P. W. Sternberg, E. Gong, F. C. Schroeder and H. Lu, Lab Chip, 2011, 11, 3689–3697 RSC.
- G. J. Lieschke and P. D. Currie, Nat. Rev. Genet., 2007, 8, 353–367 CrossRef CAS.
- B. R. Ellerbrock, E. M. Coscarelli, M. E. Gurney and T. G. Geary, J. Biomol. Screening, 2004, 9, 147–152 CrossRef CAS.
- K. Chung, M. M. Crane and H. Lu, Nat. Methods, 2008, 5, 637–643 CrossRef CAS.
- D. M. Raizen and L. Avery, Neuron, 1994, 12, 483–495 CrossRef CAS.
- R. J. Hobson, V. M. Hapiak, H. Xiao, K. L. Buehrer, P. R. Komuniecki and R. W. Komuniecki, Genetics, 2006, 172, 159–169 CrossRef CAS.
- Y. Xia, E. Kim, X. M. Zhao, J. A. Rogers, M. Prentiss and G. M. Whitesides, Science, 1996, 273, 347–349 CAS.
- Y. Xia and G. M. Whitesides, Annu. Rev. Mater. Sci., 1998, 28, 153–185 CrossRef CAS.
- J. Dillon, I. Andrianakis, K. Bull, S. Glautier, V. O'Connor, L. Holden-Dye and C. James, PLoS One, 2009, 4, e8482 Search PubMed.
- J. A. Dent, M. W. Davis and L. Avery, EMBO J., 1997, 16, 5867–5879 CrossRef CAS.
- J. C. Sheriff, A. C. Kotze, N. C. Sangster and R. J. Martin, Parasitology, 2002, 125, 477–484 CrossRef CAS.
- M. W. Davis, D. Somerville, R. Y. Lee, S. Lockery, L. Avery and D. M. Fambrough, J Neurosci, 1995, 15, 8408–8418 CAS.
- J. A. Dent, M. M. Smith, D. K. Vassilatis and L. Avery, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 2674–2679 CrossRef CAS.
- A. J. Wolstenholme and A. T. Rogers, Parasitology, 2005, 131 Suppl, S85–95 CAS.
- D. J. Pemberton, C. J. Franks, R. J. Walker and L. Holden-Dye, Mol Pharmacol, 2001, 59, 1037–1043 CAS.
- A. Crisford, C. Murray, V. O'Connor, R. J. Edwards, N. Kruger, C. Welz, G. von Samson-Himmelstjerna, A. Harder, R. J. Walker and L. Holden-Dye, Mol. Pharmacol., 2011, 79, 1031–1043 CrossRef CAS.
- P. R. Towers, B. Edwards, J. E. Richmond and D. B. Sattelle, J. Neurochem., 2005, 93, 1–9 CrossRef CAS.
- J. T. Fleming, M. D. Squire, T. M. Barnes, C. Tornoe, K. Matsuda, J. Ahnn, A. Fire, J. E. Sulston, E. A. Barnard, D. B. Sattelle and J. A. Lewis, J Neurosci, 1997, 17, 5843–5857 CAS.
- L. Avery, J Exp Biol, 1993, 175, 283–297 CAS.
- A. Pavesi, F. Piraino, G. B. Fiore, K. M. Farino, M. Moretti and M. Rasponi, Lab Chip, 2011, 11, 1593–1595 RSC.
- S. E. Hulme and G. M. Whitesides, Angew. Chem., Int. Ed., 2011, 50, 4774–4807 CrossRef CAS.
- J. E. Sulston, J. A. Hodgkin, in The nematode Caenorhabditis elegans, ed. W. B. Wood and T. C. o. C. e. Researchers, Cold Spring Harbor Laboratory Press, Plainview, NY, 1988, pp. 587–606. Search PubMed.
- W. M. Roberts, The Journal of physiology, 1987, 388, 213–232 CAS.
Footnotes |
| † Published as part of a LOC themed issue dedicated to research from the USA: Guest Editors Don Ingber and George Whitesides. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c2lc00001f |
| This journal is © The Royal Society of Chemistry 2012 |
