Materials for biosurfaces
C.
Ohm
a,
M. E.
Welch
b and
C. K.
Ober
ab
aMaterials Science & Engineering, Cornel University, Ithaca, NY 14853, USA
bChemistry and Chemical Biology, Cornel University, Ithaca, NY 14853, USA
 C. Ohm | Dr Christian Ohm studied Chemistry at the Johannes Gutenberg-University in Germany. During his Diploma thesis with Professor Wolfgang Knoll at the Max Planck Institute for Polymer Research he worked with artificial lipid bilayers. For his PhD he joined the research group of Professor Rudolf Zentel where he worked on the synthesis of liquid crystalline elastomers. Currently, he is a postdoctoral fellow in Professor Christopher Ober's research group at Cornell University. His research interests include polymer brushes and organic nanoparticles. He holds a Feodor Lynen Fellowship from the Humboldt Foundation. |
 M. E. Welch | M. Elizabeth Welch received a BA in chemistry from St Olaf College in 2008. She is currently pursuing a PhD degree at Cornell University in the research group led by Prof. Christopher K. Ober. Her research interests include the synthesis and characterization of polymer brushes relevant to direct patterning and biosensing device geometries. |
 C. K. Ober | Prof. Christopher K. Ober is the Francis Bard Professor of Materials Engineering at Cornell University. He received his B.Sc. in Honours Chemistry (Co-op) from the University of Waterloo, Ontario in 1978. He obtained his PhD in Polymer Science & Engineering from the University of Massachusetts (Amherst) in 1982. From 1982 until 1986 he was a senior staff member at the Xerox Research Centre of Canada where he worked on marking materials. Ober joined Cornell University as an Assistant Professor in the Department of Materials Science and Engineering in 1986. He recently served as Interim Dean of the College of Engineering. He has pioneered new methods in photolithography and studies the biology materials interface. His awards include the 2009 Gutenberg Research Award from the University of Mainz, the 1st Annual FLEXI Award in the Education Category (for flexible electronics) awarded in 2009, the 2007 Humboldt Research Prize, the 2006 ACS Award in Applied Polymer Science and the Photopolymer Science and Technology Award in 2004. He was elected an ACS Fellow in the 2009 Inaugural Class and is past President of the IUPAC Polymer Division. |
Introduction
Biosurfaces are of increasing importance as the interface between the biological and biomedical world and materials science due to their important role in applications such as sensing, cell growth substrates or disease detection and treatment (DOI: 10.1039/c2jm32016a, DOI: 10.1039/c2jm32242k, DOI: 10.1039/c2jm31737k, ref. 1 and 2). The articles considering biosurfaces found in this themed issue summarize aspects of these topics and generally fall into one or more of four basic categories (Fig. 1). The first two topics deal with means to control the attachment of biological materials such as cells, DNA and proteins to artificial surfaces. Depending on the type of application, either an attraction of biological attachment is required (for example for biomedical implants) or the prevention of the settlement of such structures (antifouling surfaces). The third category of biosurface interaction described in this themed issue deals with smart surfaces that can be switched between a bio-friendly and a bio-repellent state. This allows a controlled release of biomaterials like drugs or cells. Finally, in the forth category of reports the authors demonstrate how materials scientists can adapt principals from nature in order to produce biocompatible surfaces that find applications in many aspects of medical treatment and materials science.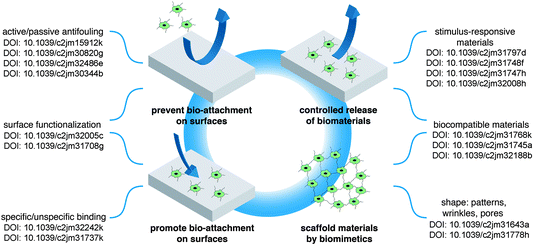 | ||
| Fig. 1 Overview over the articles found in this themed issue. | ||
Part 1: enabling controlled biological attachment
Surfaces interacting with biological systems play many important roles, but arguably the most important one is determining whether binding takes place or not. Specific binding, that is the high-affinity binding of one molecule to another, is used in natural systems for strong adhesion, identification and information transfer. Often very high equilibrium coefficients are associated with these binding events because of the unique features of the arrangement of atoms of one or both of the binding partners. Common examples of very specific interactions are antibodies binding to ligands, DNA recognition and the biotin–streptavidin system. Equilibrium affinity coefficients as high as 1014 M−1 are possible for the biotin–streptavidin system whereas for antibody-ligand binding values in the range of 107 to 109 M−1 are more common. These types of binding reactions rival covalent bonds in their permanence and specificity. In this issue, Koepsel et al. discuss the interaction of stem cells to oligopeptides bound to SAM surfaces and look for effects of specific binding on cell differentiation (DOI: 10.1039/c2jm32242k). Mey et al. use membrane-functionalized pore arrays and pore-spanning membranes on porous substrates and consider the value of large surface area for molecular recognition events (DOI: 10.1039/c2jm31737k).Part 2: preventing biological attachment
Arguably a more daunting research challenge is the prevention of non-specific binding. Any time a surface is in contact with a biological system, there is a strong prospect that undesired or uncontrolled attachment of biomolecules will occur. Biopolymers in particular are prone to binding because of the cooperative effects of very many weak forces possible from the numerous points of attachment.The problem of non-specific binding is very complex and involves many factors. It occurs not only in the medical environment, but takes place in essentially all ecosystems, and is a particular problem, for example, in the marine environment where it leads to fouling. Typically non-specific binding can be dealt with using techniques that coat surfaces with molecules such as PEG.1 This acts as an excellent barrier to both protein and cell binding to most surfaces used in biomedical applications. In part, these surfaces act to prevent the deposition of extracellular protein that can enhance cell attachment.2 Because there are so many possible additional sources of biofouling in the marine environment, this complexity provides a much more challenging situation. Both polar (e.g. PEG) and non-polar (e.g. silicone) materials can easily foul in this situation.
A variety of surface structures have been investigated for fouling resistance including self-assembled monolayers (SAMs), polymer brushes, and dendritic surfaces. In addition the composition of fouling resistant surfaces have been constructed to be non-polar, polar, amphiphilic, and charged. And with the advent of click chemistry surfaces may be easily modified with chemical functions such as peptides and other biomolecular compounds. In certain circumstances, antimicrobial surfaces are used to prevent short-term bioaccumulation.
For example, in this themed issue Kim et al. show the ability of polymer brushes modified with sulfobetaine to suppress bacterial attachment (DOI: 10.1039/c2jm15912k). Brushes include polymers attached to surfaces by various means including grown from and attached to types. Brushes typically extend 10's of nanometers from the surface and, if a dense brush, cover the surface with partially extended polymer chains. Such materials can be especially good at preventing non-specific binding and still enable functional group attachment.3
Rühe discusses the importance and use of polymer brush networks for their ability to resist protein adhesion, the first step in surface fouling, using fibrinogen as a test material. In this study polymer chains were both attached and crosslinked using benzophenone photoradical reaction (DOI: 10.1039/c2jm30820g). Imbesi et al. examine the design of amphiphilic anti-fouling network surfaces using thiol–ene “click” chemistry (DOI: 10.1039/c2jm32005c).
Part 3: controlled release of biomaterials from a surface
In this issue Nagase et al. used the variable adhesion of poly(N-isopropyl acrylamide) (PNIPAm) brush grafted glass surfaces to study the attachment and release of cultured cell lines on surfaces, in effect turning a sticky surface into an anti-fouling surface through temperature dependent chain coil conformation (DOI: 10.1039/c2jm31797d). Nash et al. review PNIPAm brushes as thermoresponsive films for cell culture carriers and in cell sheet delivery and a broad range of additional applications (Fig. 2) (DOI: 10.1039/c2jm31748f). Kamperman reviews switchable adhesion triggered by temperature, phase change, light, pH and solvent change (DOI: 10.1039/c2jm31747h). In addition, topography and magnetic and electric field effects are also described. Paez et al. explore the use of polyglycerol dendrimers on gold with disulfide and amino functions and examine the fouling resistance of these surfaces (DOI: 10.1039/c2jm32486e).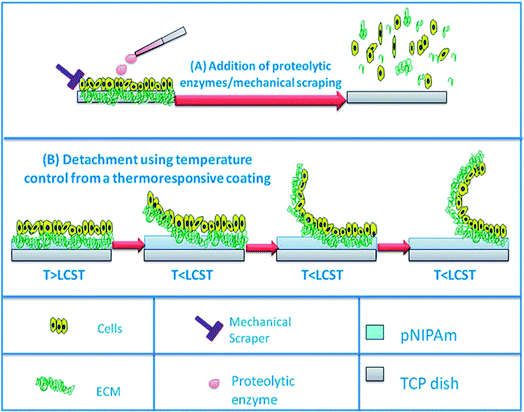 | ||
| Fig. 2 (A) Schematic illustrating the detachment of cells from substrate via conventional processes i.e. via the addition of proteolytic enzymes or mechanical scraping. (B) Schematic illustrating the detachment of cells. For more details, see Nash et al., this issue (DOI: 10.1039/c2jm31748f). | ||
Another reason to favorably interface with biological media is the potential for active substance release. Common delivery systems consist of the release of diagnostic and pharmaceutical agents (nutrients, drugs, vitamins etc.), but other significant applications include food packaging, self-healing, and active coatings (DOI: 10.1039/c2jm32486e and ref. 4–9). Depending on the environment and function, different techniques of distribution can be applied.
When a cell is released from a surface, harmful factors can result such as damage of the cellular membrane and cleavage of important transmembrane proteins, both of which can lead to the loss of cellular function. One method shown to circumvent these problems is the use of temperature mediated detachment. Poly-N-isopropylacrylamide (pNIPAm) is one of the most commonly studied thermoresponsive systems due to its ability to reversibly switch from a hydrophilic to hydrophobic state in a physiologically relevant temperature range (32 °C). The change between its swollen and collapsed state is an entropy effect attributed to hydrogen-bond formation with water and intramolecular hydrophobic forces (DOI: 10.1039/c2jm31748f). Another type of thermoresponsive coating is a copolymer of 2-(2-methoxyethoxy)ethyl methacrylate and oligo(ethylene glycol) methacrylate (P(MEO2MA-co-OEGMA)). Reported by Lutz et al. and Hu et al., these polymeric materials also undergo thermoreversible volume-phase transitions at a temperature range similar to that of pNIPAm, and can also form crystalline structures.10,11 The nontoxic and anti-immunogenic properties of PEG demonstrate the potential use of this polymer in biomedical applications.12
Activating the matrix surrounding desirable biomolecules via electrochemical stimulation is another method currently being investigated. This technique, which is usually comprised of biodegradable polymers, eliminates biomolecule exposure to harsh organic environments, high temperatures and physical strain. Katz et al. have demonstrated the use of alginate gels cross-linked with Fe3+ cations to release the enzyme lysozyme when electrochemically induced (DOI: 10.1039/c2jm32008h). Iron cations in the Fe3+ state act as crosslinkers to form an alginate hydrogel that can encase lysozyme. When a reducing potential is applied, the iron converts to its Fe2+ state which can only weakly interact with alginate thus allowing the film to dissolve and release the enzyme. Other suitable materials include the conducing polymer polypyrrole (PPy). To release drug molecules, PPy can easily be converted to its reduced form when a negative potential is applied. Studies have demonstrated the use of PPy to release therapeutic reagents such as neurotrophins,13 proteins,14 dexamethasone,15 and antischistosomiasis compounds.16
Some delivery systems utilize the fact that the human body has a diverse pH range and thus controlled release is possible in different locations of the drug administration route. Hydrogels have been extensively studied for this application due to their pH-responsive properties. When in the swollen state, they resemble biological tissue and can retain the absorbed solution until activated by environmental stimuli. A wide range of materials are available which have properties including non-toxicity, biocompatibility, and biodegradability making these systems an excellent choice for drug delivery methods (DOI: 10.1039/c2jm31745a and ref. 17–19).
Part 4: biocompatible materials by biomimetics
The fourth section of this themed issue deals with the concept of mimicking nature in order to obtain smart and biocompatible surfaces. The resulting materials find application in medical treatment (wound healing, tissue replacement, drug delivery) and materials science concepts (surface patterning, alignment, encapsulation and controlled release).One characteristic of biological materials is the rarity of smooth surfaces. Natural objects like organs, leaves or fruit are wrinkled, creased or folded (Fig. 3). In his review article, Ionov explains, how nature can generate such complex shapes by applying concepts like inhomogeneous growth or drying processes (DOI: 10.1039/c2jm31643a). Based on these insights, he reviews several examples of artificial structures that possess the same complexity in shape. The underlying principle for making these structures is that two different kinds of polymeric materials are laminated into one object (thin films or nanoparticles). The deformation of one component (expansion or contraction) leads to self-assembling phenomena into various shapes.
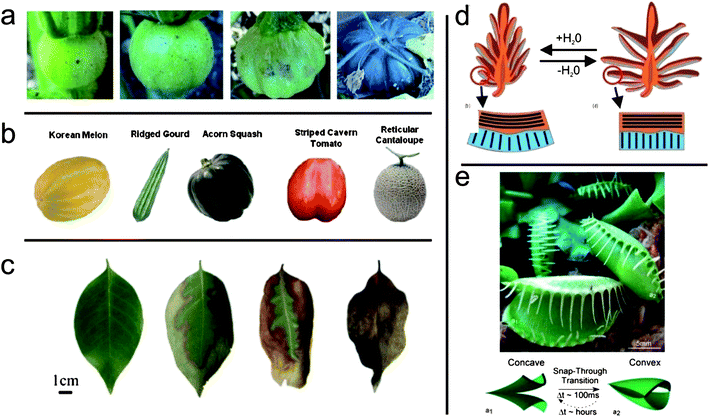 | ||
| Fig. 3 Complex shapes found in nature. (a) Growth of a pumpkin (Copyright (2009) by Elsevier) (b) several fruits (Copyright (2009) by Elsevier) (c) dehydration of a leaf (d) opening of a pine cone (Copyright (2011) by Elsevier) (e) movement of the Venus flytrap (Copyright (2007) by Wiley-VCH Verlag GmbH & Co. KGaA). See Ionov, this issue (DOI: 10.1039/c2jm31643a). | ||
Other natural materials like bones or egg-shells owe their combination of stability and lightness to their micro- or nanoporosity. In their article, Minko et al. present the fabrication of nanoporous hydrogel films from ion-crosslinked sodium alginate (DOI: 10.1039/c2jm31778h). For this, they spin-coated mixtures from sodium alginate and poly(vinyl alcohol) on silicone wafers. Immersing those films in a calcium chloride solution led to gelation of the alginate as well as dissolution of the phase separated poly(vinyl alcohol), yielding porous hydrogel films with pore sizes in the nanometer size region.
In another article by Janshoff (DOI: 10.1039/c2jm31737k) the authors review the use of inorganic nanoporous materials as hosts for artificial cellular membranes. These pore-spanning lipid bilayers serve as model systems for the study of transport phenomena in living cells. Other possible applications are biosensors and protein purification.
Besides mimicking natural shapes, researchers have also rebuilt the complex chemical composition of biological materials. Heilshorn et al. present an article about the preparation of free-standing films from photocrosslinked, cell-adhesive elastin-like proteins (DOI: 10.1039/c2jm31768k). The proteins were made by a standard recombinant synthesis in an E. coli host followed by chemical functionalization of the lysine amino acids with a photocrosslinkable group. These modified proteins were processed into various shapes by spin coating or soft molding and were crosslinked by UV-irradiation. The resulting films were mechanically robust and the authors discussed possible biomedical applications.
Another example for applying natural materials to obtain complex functionality is provided by Tirrell et al. The authors prepared conjugates from peptide sequences with fatty acids (peptide amphiphiles) (DOI: 10.1039/c2jm31745a). Depending on environmental parameters, solutions of these materials had very different properties. At a low pH, they were viscoelastic liquids, making them injectable. However, at a pH above 6.5 they transitioned into self supporting hydrogels with high moduli. Thus, they are well suited for applications as tissue scaffolds.
Concluding comment
This collection of articles attempts to provide a summary of the current state of understanding of materials for biosurfaces. The rate at which new developments take place on this topic is remarkable and with the number of new examples that are reported every day there is a risk of rapid aging of these articles. It is our hope that the choice of the topics and the selection of articles will not only ensure stimulating reading but remain current and helpful to the reader for the foreseeable future.Acknowledgements
The authors would like to thank the Humboldt Foundation for a Feodor Lynen Fellowship (CO) and a Senior Research Prize (CKO). We are also grateful to the National Science Foundation for grant DMR-1105253 that supports one of us (MEW) and has enabled our involvement in this area of research.References
- W. Senaratne, L. Andruzzi and C. K. Ober, Biomacromolecules, 2005, 6, 2427–2448 CrossRef CAS.
- E. N. Chiang, R. Dong, C. K. Ober and B. A. Baird, Langmuir, 2011, 27(11), 7016–7023 CAS.
- M. E. Welch, A. Rastogi and C. K. Ober, Soft Matter, 2011, 7(2), 297–302 RSC.
- L. Di, X. S. Jin and H. C. Zhi, Sci. China: Chem., 2010, 53, 612–618 CrossRef.
- V. P. Torchilin, J. Controlled Release, 2001, 73, 137–172 CrossRef CAS.
- M. R. Moreira, M. Pereda, N. E. Marcovich and S. I. Roura, J. Food Sci., 2011, 76, 54–63 CrossRef.
- G. G. Buonocore, M. A. Del Nobile, A. Panizza, S. Bove, G. Battaglia and L. Nicolais, J. Food Sci., 2003, 68, 1365–1370 CrossRef CAS.
- T. Traitel, R. Goldbart and J. Kost, J. Biomater. Sci., Polym. Ed., 2008, 19, 755–767 CrossRef CAS.
- D. G. Shchukin, D. O. Grigoriev and H. Möhwald, Soft Matter, 2010, 6, 720–725 RSC.
- J. F. Lutz, J. Andrieu, S. Üzgün, C. Rudolph and S. Agarwal, Macromolecules, 2007, 40, 8540–8543 CrossRef CAS.
- T. Cai, M. Marquez and Z. Hu, Langmuir, 2007, 23, 8663–8666 CrossRef CAS.
- M. Bikram and J. L. West, Expert Opin. Drug Delivery, 2008, 5, 1077–1091 CrossRef CAS.
- B. C. Thompson, S. E. Moulton, R. T. Richardson and G. G. Wallace, Biomaterials, 2010, 32, 3822–3831 CrossRef.
- G. Jeon, S. Y. Yang, J. Byun and J. K. Kim, Nano Lett., 2011, 11, 1284–1288 CrossRef CAS.
- X. Luo and X. T. Cui, Electrochem. Commun., 2009, 11, 1956–1959 CrossRef CAS.
- Y. Li, R. J. Ewen, S. A. Campbell and J. R. Smith, J. Mater. Chem., 2012, 22, 2687–2694 RSC.
- F. Li, Y. Zhu, B. You, D. Zhao, Q. Ruan, Y. Zeng and C. Ding, Adv. Funct. Mater., 2010, 20, 669–676 CrossRef CAS.
- P. Sun, P. Li, Y. Li, Q. Wei and L. Tian, J. Biomed. Mater. Res., Part B, 2011, 97B, 175–183 CrossRef CAS.
- Q. Wang, J. Zhang and A. Wang, Carbohydr. Polym., 2009, 78, 731–737 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
