Micron- and nanosized FAU-type zeolites from fly ash for antibacterial applications†
Lubomira
Tosheva
*a,
Ava
Brockbank
a,
Boriana
Mihailova
b,
Justyna
Sutula
a,
Joachim
Ludwig
b,
Herman
Potgieter
a and
Joanna
Verran
a
aFaculty of Science and Engineering, Manchester Metropolitan University, Chester Street, Manchester, M1 5GD, UK. E-mail: l.tosheva@mmu.ac.uk; Fax: +44 (0)161 2476840; Tel: +44 (0)161 2471426
bMineralogisch-Petrographisches Institut, Universität Hamburg, Grindelallee 48, D-20146 Hamburg, Germany
First published on 22nd June 2012
Abstract
The development of procedures for the synthesis of nanosized zeolites from cheap sources in the absence of organic templates is of great industrial importance. In this work, the size of FAU-type zeolites was decreased from micron- to nano-dimensions by reducing the amount of distilled water added to fly ash after alkali fusion. The fly ash zeolites showed high antimicrobial activity after introduction of Cu and Ag, which was associated with the release of Cu/Ag into the medium. This activity of Cu-containing zeolites from fly ash was similar to the activity of Cu-containing zeolites prepared by conventional methods but lower than that of Ag-containing zeolites.
1 Introduction
The reduction of the size of zeolite crystals from micron- to nano-dimensions leads to an increase in the external surface area, short diffusion paths and can increase life-time.1–5 Nanozeolites in the form of colloidal suspensions of discrete nanoparticles with narrow particle size distributions have been prepared in the presence of excessive amounts of organic structure-directing templates at low yields. Owing to the colloidal nature of these zeolites and the numerous methods used to manipulate the nanoparticles, colloidal zeolites have been extensively used for the preparation of structured 2D- and 3D-materials, both supported and unsupported. For many traditional applications of zeolites, such as catalysis and sorption processes, the discrete nature of the zeolite nanocrystals may not be of paramount importance and aggregates of nanosized particles can still offer superior performance compared to microcrystalline powders. FAU-type zeolites are amongst the most important commercial catalysts6 and therefore the development of economical and environmentally favourable methods for synthesis of nanosized FAU-type zeolites is of industrial interest. The main approach to meet both criteria is to perform the syntheses in the absence of organic templates. Additional requirements include the use of cheap silica and alumina sources and minimized waste solutions. Aggregates of FAU-type nanosized crystals in the absence of organic templates have been prepared from highly reactive gels at room temperature,7via a three-stage temperature control synthesis procedure,8 and by optimising the experimental conditions using 23 factorial methods.9 In this study, we utilised fly ash (FA) as a cheap raw material for the synthesis of FAU-type zeolites of controlled crystal size, including aggregates of nanosized particles, in the absence of organic templates.Fly ash is a waste material formed in coal-fired power plants. Despite the increasing utilization of fly ash, mainly in the building and construction industry, there are still millions of tons of disposed fly ash around the world every year. The recycling of fly ash into zeolites offers an excellent solution to the fly ash disposal problem.10 Conversion of FA to zeolites has been achieved by hydrothermal treatment of FA after pre-activation with NaOH solutions.11–17 However, the resultant products were generally not fully crystalline, contained impurities of other crystalline and amorphous phases, and the zeolite crystallised on the surface of the partially dissolved micron-sized spherical FA particles. Alkali fusion synthesis has been developed to improve the phase purity and degree of crystallinity of zeolites prepared from fly ash.18–20 The effect of various parameters, such as the FA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaOH weight ratio, the fusion temperature, and the crystallisation temperature and time, on the characteristics of the product zeolites has been extensively studied in these works. Here, we show that the size of FAU-type zeolites prepared from FA by alkali fusion can be controlled by the amount of water added to the fused FA prior to hydrothermal treatment. The zeolitisation process leading to the formation of micron- and nanosized FAU-type zeolites was studied by X-ray diffraction, Raman spectroscopy, and gas adsorption. The antimicrobial activity of zeolites loaded with metals such as silver and copper has been demonstrated in a number of studies.21–26 Further objectives of the present work were to evaluate the antibacterial activity of the fly ash zeolites after loading with copper or silver in comparison with a reference zeolite prepared by conventional hydrothermal treatment as well as depending on the size of the fly ash zeolite crystals. The Gram-negative Escherichia coli (E. coli) and Pseudomonas aeruginosa (Ps. aeruginosa) and the Gram-positive Enterococcus faecalis (Ent. faecalis) bacteria associated with faecal contamination of drinking water (E. coli, Ent. faecalis) or with the general cleanliness of water distribution systems (Ps. aeruginosa) were selected for the antimicrobial tests.27
NaOH weight ratio, the fusion temperature, and the crystallisation temperature and time, on the characteristics of the product zeolites has been extensively studied in these works. Here, we show that the size of FAU-type zeolites prepared from FA by alkali fusion can be controlled by the amount of water added to the fused FA prior to hydrothermal treatment. The zeolitisation process leading to the formation of micron- and nanosized FAU-type zeolites was studied by X-ray diffraction, Raman spectroscopy, and gas adsorption. The antimicrobial activity of zeolites loaded with metals such as silver and copper has been demonstrated in a number of studies.21–26 Further objectives of the present work were to evaluate the antibacterial activity of the fly ash zeolites after loading with copper or silver in comparison with a reference zeolite prepared by conventional hydrothermal treatment as well as depending on the size of the fly ash zeolite crystals. The Gram-negative Escherichia coli (E. coli) and Pseudomonas aeruginosa (Ps. aeruginosa) and the Gram-positive Enterococcus faecalis (Ent. faecalis) bacteria associated with faecal contamination of drinking water (E. coli, Ent. faecalis) or with the general cleanliness of water distribution systems (Ps. aeruginosa) were selected for the antimicrobial tests.27
2 Experimental
2.1 Zeolite synthesis
Fly ash (FA, 10 g) from the Lethabo Power Station (SuperPozz, South Africa) was ground with NaOH pellets (BDH, ≥99%, 12 g) and fused at 600 °C for 3 h. The fused fly ash (FFA) was ground again followed by the addition of distilled water at two solid to water weight ratios, namely 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and ageing with agitation for 68 h in polypropylene reactors. In addition, zeolite precursor samples were prepared for shorter ageing times, and labelled as PFAZ4-Nh or PFAZ10-Nh, correspondingly, where N is the treatment time (in hours). After 68 h of stirring, the stirrer bars were removed and the reactors were transferred to a preheated oven at 80 °C. The samples were hydrothermally treated for 26 h, filtered, rinsed with distilled water and dried at room temperature. The products were denoted as FAZ4 and FAZ10, respectively. A reference FAU-type zeolite (ZRef) was prepared by conventional hydrothermal treatment of a gel with the molar composition 8NaOH
10, and ageing with agitation for 68 h in polypropylene reactors. In addition, zeolite precursor samples were prepared for shorter ageing times, and labelled as PFAZ4-Nh or PFAZ10-Nh, correspondingly, where N is the treatment time (in hours). After 68 h of stirring, the stirrer bars were removed and the reactors were transferred to a preheated oven at 80 °C. The samples were hydrothermally treated for 26 h, filtered, rinsed with distilled water and dried at room temperature. The products were denoted as FAZ4 and FAZ10, respectively. A reference FAU-type zeolite (ZRef) was prepared by conventional hydrothermal treatment of a gel with the molar composition 8NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2Al2O3
0.2Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0SiO2
1.0SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200H2O at 110 °C for 3 h.7
200H2O at 110 °C for 3 h.7
2.2 Metal-loaded zeolites
The FAZ samples were suspended in 0.05 M Cu(NO3)2 (copper(II) nitrate hemi(pentahydrate), 98%, Alfa Aesar) or 0.01 M AgNO3 (silver nitrate, 99.9+%, Alfa Aesar) solutions at a solid to solution ratio of 1 to 100, and stirred for 48 h. The resultant Me-zeolite samples (Me-FAZ4 and Me-FAZ10, where Me is Cu or Ag) were filtered, rinsed with distilled water and dried at room temperature. All the above procedures when silver was used were performed in the dark by covering the glassware and sample vials with aluminium foil. Cu-ZRef was prepared analogously to the Cu-FAZ samples.2.3 Characterisation
Powder X-ray diffraction (XRD) measurements were performed with a Philips X′Pert diffractometer (Bragg–Brentano geometry), using Cu Kα radiation. XRD patterns were collected in a 2θ range from 3 to 70°, with a step size of 0.02° and an accumulation time of 2 s per step. Raman spectra were collected in the spectral range 15–4000 cm−1 with a Horiba Jobin-Yvon T64000 triple-grating spectrometer equipped with an Ar+ laser (Coherent 90C FreD), an Olympus BH41 microscope and a liquid-nitrogen-cooled CCD detector Symphony. The measurements were conducted in a backscattering geometry, using an incident laser wavelength of 514.5 nm, a power density on the sample surface between 3.0 and 0.15 kW mm−2 and corresponding acquisition times between 60 and 360 s, depending on the sample stability under the laser irradiation, at a spectral resolution of 2 cm−1. All measured spectra were reduced by the Bose–Einstein occupation factor to eliminate the effect of temperature on the peak intensities. The morphology of the samples was studied by Scanning Electron Microscopy (SEM) using a JEOL 5600LV instrument. Semi-quantitative chemical analysis was performed by energy-dispersive X-ray spectroscopy (EDS) using a detector from Oxford Instruments. The powders were pressed into pellets and coated with C prior to analysis. An area of ca. 150 × 180 μm was scanned using a voltage of 10 keV, working distance of 20 mm and 30 s acquisition times. The average elemental composition (wt%) based on three scanned areas for each sample was calculated using the INCA Software. The chemical analysis of the raw fly ash was performed on a Bruker SRS 3300 XRF spectrometer after fusion with a lithium tetraborate flux.28 Nitrogen adsorption isotherms were recorded on a Micromeritics ASAP 2020 surface area analyser at −196 °C. Samples were degassed at 350 °C for 12 h prior to analysis. Specific surface areas were calculated using the BET equation in the 0.05–0.3 range of relative pressure, whereas external surface areas and micropore volumes were determined by the t-plot method. Pore-size distributions were determined by the BJH method from the desorption branches of the isotherms.2.4 Antibacterial testing
The microbial cultures were prepared by inoculating a single colony from a freshly cultured agar plate into 100 ml of sterile broth. Tryptic soya agar broth (Oxoid) was used for E. coli (strain NCTC 9001), brain–heart infusion agar liquid medium (Oxoid) for Ent. faecalis (strain NCTC 775) and nutrient agar broth for Ps. aeruginosa (strain NCTC 6749). All liquid cultures were incubated aerobically at 37 °C for 18 hours on a rotary shaker at 150 rpm. Standardised cell suspensions were prepared by centrifugation of the cultures at 3000 rpm for 10 minutes to form a pellet. The supernatant was discarded and the pellet was re-suspended in 100 ml of sterile distilled water. The approximate starting concentrations of the bacterial suspensions were 109 colony forming units (CFU) per ml.Initial experiments were performed with the Cu-loaded zeolites and E. coli. Firstly, different amounts of the Cu-ZRef sample were used to obtain preliminary data on the antibacterial activity of the sample. 0.03 g, 0.01 g, and 0.005 g Cu-ZRef samples were sterilised by autoclaving at 121 °C for 15 min. 20 ml of sterile distilled water were added to the zeolite followed by the addition of 100 μl of the standardised bacterial suspension. The mixtures were incubated at 37 °C under gentle stirring. Control experiments using copper-free zeolites (negative control) and experiments in the absence of any zeolite (positive control) were also performed. 500 μl samples were taken at different time intervals and each sample was serially diluted ten-fold in the range 10−1 to 10−6. Each diluted sample (100 μl) was plated onto duplicate plates of tryptic soya agar for culturing E. coli and incubated overnight at 37 °C in an aerobic atmosphere. The number of colonies was counted and the average CFU per ml was calculated. Similar experiments were performed using 0.03 g Cu-FAZ samples.
The antibacterial properties of the Ag-FAZ zeolites were then studied in detail using all three microorganisms and 0.01 g of Ag-FAZ as described above, but in the dark by covering with aluminium foil throughout the tests. The antimicrobial activity of the Ag-FAZ samples was measured every 15 min over 1 h of exposure. Each dilution (100 μl) was plated onto duplicate plates of tryptic soya agar for culturing E. coli, brain–heart infusion agar for Ent. faecalis and nutrient agar for Ps. aeruginosa. After overnight incubation at 37 °C in aerobic (E. coli and Ps. aeruginosa) and CO2-enriched atmospheres (Ent. faecalis), the number of colonies was counted and the average number of CFU per ml was calculated. The antibacterial potential of Ag-FAZ10 was further studied by adding an additional fresh E. coli inoculum after the first 1 h of exposure, and the sampling continued over another 1 h of exposure. In another series of experiments, the Ag-FAZ4 and Ag-FAZ10 antibacterial effects were studied semi-quantitatively over a short time period using E. coli and Ent. faecalis. For these tests, the zeolite suspensions were prepared as described above and the corresponding microorganisms were added. A sample was then taken every 30 s using a sterile loop for up to 60 min. A loopful of sample was streaked over an eighth of a plate of tryptic soya agar for culturing E. coli, and brain–heart infusion agar for Ent. faecalis. The amount of growth was assessed visually after overnight incubation at 37 °C in an aerobic (E. coli) or CO2-enriched atmosphere (Ent. faecalis). All 15 min and 30 s experiments were repeated twice.
The release of copper and silver from the zeolite samples was measured in the mother liquor after 1 h of incubation. The supernatants were analyzed on a Varian Vista AX CCD inductively coupled plasma atomic emission spectrometer (ICP-AES) using the Cu 324.8 nm or the Ag 328.1 nm analytical wavelengths. Plasma viewing occurred in the axial mode, and the following operating conditions were used in the spectrometer: a forward power of 1200 W, an Ar plasma flow of 15 l min−1, an auxiliary flow of 1.5 l min−1, a nebuliser flow of 0.75 l min−1, a glass cyclonic spray chamber, and a glass nebuliser.
3 Results and discussion
3.1 Conversion of fly ash to FAU-type zeolites
The chemical composition of the raw FA is given in Table 1 and EDS data for the zeolite samples prepared in this work are provided in Table 2. The raw fly ash consisted of spherical particles with a broad particle size distribution in the range 1–20 μm (Fig. 1a). 2–5 μm crystals with the typical octahedral morphology of a FAU-type zeolite were observed in the SEM images of the ZRef sample (Fig. 1d). The FAZ4 sample was composed of aggregates of nanosized particles with the size of the primary particles ranging from 50 nm to 400 nm (Fig. 1c). The size of the majority of the aggregated particles was about 1.5–5 μm, although larger aggregates up to 15 μm were also observed (Fig. 1b). The latter did not resemble the spherical morphology of the fly ash (Fig. 1a) confirming the results from previous studies that alkali fusion improved the solubility of fly ash.18–20 The FAZ10 sample consisted of relatively uniform, ca. 3 μm FAU-type crystals (Fig. 1e and f), which were similar to the ZRef crystals (Fig. 1d).| ZRef | FAZ4 | FAZ10 | Cu-ZRef | Cu-FAZ4 | Cu-FAZ10 | Ag-FAZ4 | Ag-FAZ10 | |
|---|---|---|---|---|---|---|---|---|
| Si/Al | 1.3 | 1.4 | 1.4 | 1.3 | 1.3 | 1.5 | 1.4 | 1.4 |
| Na | 12.4 | 11.3 | 10.9 | 1.4 | — | — | 5.3 | 5.9 |
| Ca | — | 2.2 | 2.5 | — | — | — | 2.2 | 2.3 |
| Cu/Ag | — | — | — | 16.2 | 17.4 | 17.3 | 15.2 | 13.9 |
 | ||
| Fig. 1 SEM images of (a) the initial fly ash, (b and c) FAZ4, (d) ZRef, and (e and f) FAZ10 samples. | ||
The XRD patterns of the initial fly ash, selected samples prepared at different stages of the zeolite synthesis from fly ash and the reference zeolite sample are shown in Fig. 2. The FA contained an amorphous phase, mullite (Al6Si2O13, ICDD pdf number 000-15-0776) and quartz (SiO2, ICDD pdf number 000-46-1045). The amount of amorphous material increased considerably after alkali fusion as seen from the large amorphous halo in the XRD pattern of FFA. Further, the intensity of the quartz and mullite peaks decreased and two new crystalline phases were identified, calcite (Ca(CO3), ICDD pdf number 000-83-1762) and sodium aluminium silicate (Na8Al4Si4O18, ICDD pdf number 000-76-2386). According to XRD analysis, the structures of the FFA and PFAZ samples were similar. The XRD pattern of the ZRef sample was typical of a well-crystalline FAU-type zeolite (Faujasite-Na, ICDD pdf number 000-12-0246). The FAU-type phase was the main phase in both FAZ samples. However, a dense sodium aluminium silicate phase (Na12Al12Si12O48, ICDD pdf number 000-83-2151) as well as quartz impurities were also present in both FAZ samples with more pronounced peaks in the XRD pattern of FAZ4 compared to FAZ10. The XRD pattern of FAZ10 was similar to that of ZRef indicating that the sample was highly crystalline. The peaks in the XRD pattern of FAZ4 were broadened in agreement with the decreased particle size observed by SEM.
 | ||
| Fig. 2 XRD patterns of fly ash (FA), fly ash after alkali fusion (FFA), precursor zeolite prior to hydrothermal treatment (PFAZ), fly ash zeolites (FAZ) and zeolite reference (ZRef) samples. M: mullite; Q: quartz; Ca: calcite; *: sodium aluminium silicate phases. | ||
Nitrogen adsorption measurements were further used to obtain information about the pore structure of the samples prepared in this work. The isotherm of the FA was type II isotherm typical of non-porous solids (Fig. S1, ESI†).29 A representative isotherm of the PFAZ samples is shown in Fig. 3. All PFAZ isotherms were similar independently of the water content and ageing time and no conclusions about the influence of these parameters on the pore structure of the precursor samples could be drawn. The isotherms were type IV indicating the presence of mesopores, with a type H3 hysteresis loop, which is characteristic of adsorbents with plate-like particles giving rise to slit-shaped pores.29 The BET surface area changed from 1.8 m2 g−1 for FA to 20–35 m2 g−1 for the PFAZ samples, mainly due to an increase in the external surface area of the latter (Table 3). The isotherms of FAZ4 and FAZ10 indicated the presence of approximately the same amount of microporous material as evident from the similar amounts of volume adsorbed at low, <0.1 relative pressure, and confirmed by the similar micropore volumes (Table 3). This and the increased external surface area of FAZ4 suggest that the broadening of the XRD peaks corresponding to the FAU-type zeolite in the XRD pattern of FAZ4 is indeed due to a decrease in the particle size. The steepness in the increase of the volume adsorbed as the relative pressure increased decreased in the order FAZ4 > FAZ10 > ZRef. Also, there was a difference in the hysteresis loops for the zeolite samples at high relative pressures. The hysteresis loop of FAZ4 was type H3 similar to the PFAZ samples. The hysteresis loop of the FAZ10 isotherm was, however, different and similar to that of ZRef. These results were further confirmed by the BJH pore-size distribution plots (Fig. S2, ESI†). The BJH plots of the PFAZ10-27h and FAZ4 samples were similar to each other and differed from the plot for the FAZ10 sample.
The nitrogen adsorption analysis showed that the water content had a little effect on the pore structure of the precursor zeolite samples from fly ash and the main reorganizations within the pore structure occurred during the hydrothermal treatment. During the hydrothermal treatment, microporosity developed in the PFAZ4 sample but the mesopore structure of FAZ4 remained similar to the precursor. In the case of PFAZ10, both the micropore and mesopore structures changed as a result of the formation of well-defined micron-sized FAU-type crystals. Raman spectroscopy was used to study the structure of different samples prepared in the course of the zeolitization process to elucidate the role of water. The Raman scattering of FA, FFA and the intermediate and final products of the fly ash zeolitisation using a solid to water weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 is depicted in Fig. 4. The Raman spectrum of ZRef is also included in this figure as a reference. The typical Raman scattering collected from FA did not correspond to that of mullite30 or quartz,31 which were the main crystalline phases detected by XRD. The Raman spectrum of FA consisted of intense broad peaks in the range 200–600 cm−1 typical of silica and alumosilicate glass.32,33 It also contained a broad low-wavenumber feature at 85 cm−1 known as the boson peak characteristic of amorphous matter.32,33 Hence, the Raman spectrum of FA along with the FA chemical composition (Table 1) indicated that a framework Al–Si–O glass was the dominant phase in FA. The glassy structure of FFA substantially differed from that of FA. FFA exhibited considerable structural inhomogeneity as revealed by the difference in the Raman spectra collected from different areas. However, common features in all FFA Raman spectra were the bump near 850 cm−1, which most probably arose from SiO4 oligomers,34 and the sharp peaks in the range 900–1100 cm−1, which may be attributed to libration modes of OH groups.35 PFAZ10-17h contained calcite (the sharp peak at 1085 cm−1),36 the fraction of which increased with time but calcite was not detected in the Raman spectra of FAZ10. Stirring in distilled water enhanced the connectivity in the amorphous silicate–aluminosilicate system, as indicated by the reappearance of the boson peak in the spectrum of PFAZ10-17h. Raman spectra collected from different areas of PFAZ10 samples revealed that the structural homogeneity improved as well with ageing. In addition, the Raman scattering of the PFAZ-17h in the range 450–600 cm−1 was similar to that of FAU-type zeolites, indicating a resemblance of the structure on the mesoscopic scale.
10 is depicted in Fig. 4. The Raman spectrum of ZRef is also included in this figure as a reference. The typical Raman scattering collected from FA did not correspond to that of mullite30 or quartz,31 which were the main crystalline phases detected by XRD. The Raman spectrum of FA consisted of intense broad peaks in the range 200–600 cm−1 typical of silica and alumosilicate glass.32,33 It also contained a broad low-wavenumber feature at 85 cm−1 known as the boson peak characteristic of amorphous matter.32,33 Hence, the Raman spectrum of FA along with the FA chemical composition (Table 1) indicated that a framework Al–Si–O glass was the dominant phase in FA. The glassy structure of FFA substantially differed from that of FA. FFA exhibited considerable structural inhomogeneity as revealed by the difference in the Raman spectra collected from different areas. However, common features in all FFA Raman spectra were the bump near 850 cm−1, which most probably arose from SiO4 oligomers,34 and the sharp peaks in the range 900–1100 cm−1, which may be attributed to libration modes of OH groups.35 PFAZ10-17h contained calcite (the sharp peak at 1085 cm−1),36 the fraction of which increased with time but calcite was not detected in the Raman spectra of FAZ10. Stirring in distilled water enhanced the connectivity in the amorphous silicate–aluminosilicate system, as indicated by the reappearance of the boson peak in the spectrum of PFAZ10-17h. Raman spectra collected from different areas of PFAZ10 samples revealed that the structural homogeneity improved as well with ageing. In addition, the Raman scattering of the PFAZ-17h in the range 450–600 cm−1 was similar to that of FAU-type zeolites, indicating a resemblance of the structure on the mesoscopic scale.
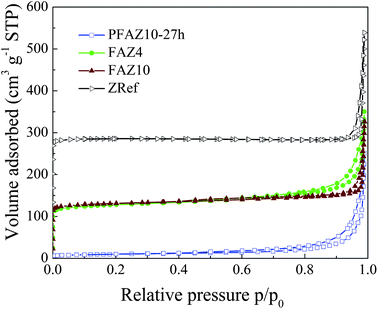 | ||
| Fig. 3 Nitrogen adsorption–desorption isotherms at −196 °C of PFAZ10-27h, FAZ4, FAZ10 and ZRef samples. The ZRef isotherm is shifted by 100 along the Y-axis for clarity. | ||
| FA | PFA10-27h | FAZ10 | FAZ4 | ZRef | |
|---|---|---|---|---|---|
| SA (m2 g−1) | 1.8 | 33.4 | 441.6 | 434.5 | 554.2 |
| V micro (cm3 g−1) | 0.000 | 0.003 | 0.175 | 0.162 | 0.280 |
| SAEXT (m2 g−1) | 1.4 | 27.1 | 63.2 | 82.6 | 16.2 |
 | ||
| Fig. 4 Raman spectra of the fly ash (FA), NaOH, fused fly ash (FFA), PFAZ10, FAZ10 and ZRef. | ||
Raman spectra of PFAZ4 and FAZ4 samples are shown in Fig. 5. The Raman scattering of PFAZ4 samples collected from different areas indicated that the homogeneity of the glassy structure did not improve with ageing time for this system. This structural inhomogeneity was also translated into the FAZ4 sample as seen by the differences in the Raman spectra collected from different areas of FAZ4. In addition, the intermediate-range order of PFAZ4-27h did not match that of FAU-type zeolites. This result may suggest that the structural homogeneity of the precursors formed during ageing of the fused material in distilled water is of paramount importance for the successful formation of highly crystalline zeolite products of high structural homogeneity. The inhomogeneity of the precursor PFAZ4 samples may be due to the limited amount of water in the slurry causing the development of local areas of varying structural and chemical composition during stirring. It has been suggested before that the mechanism of zeolite crystallization from fly ash involved three stages: (i) dissolution of fly ash and formation of an amorphous aluminosilicate gel; (ii) enrichment of the gel with Al and formation of Al-rich nuclei; and (iii) crystal growth.14,17,19 Further, dissolution of fly ash has been found to increase with increasing the level of dilution, which was also accompanied with increased crystallization times.12,15 Thus, the higher water levels during ageing in the synthesis of FAZ10 resulted in more homogeneous nucleation and the development of a zeolite-like intermediate range ordering in the amorphous structure of the precursor. The differences in the local organisation of the precursor PFAZ samples resulted in different crystallisation behaviour during the hydrothermal treatment. The decrease in the size of FAU-type crystals with a decrease in the water content is well-known and can explain the larger crystal size of FAZ10.8,9 The variations in the size of the primary FAU-type crystals and crystal aggregates as well as the inferior phase purity of the FAZ4 sample could be associated to the inhomogeneous structure of the amorphous matter developed during the ageing process.
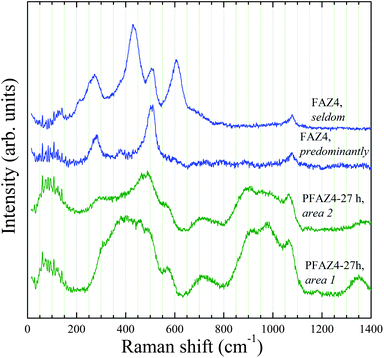 | ||
| Fig. 5 Raman spectra of PFAZ4 and FAZ4 samples. | ||
3.2 Antibacterial properties of Cu- and Ag-loaded FAU-type zeolites from fly ash
Copper and silver were ion-exchanged into the FAZ samples to introduce antibacterial activity in the fly ash zeolites. The chemical composition of the metal-loaded zeolites is given in Table 2 and XRD patterns of the Cu-FAZ and Ag-FAZ samples are provided in Fig. S3 and S4, respectively (ESI†). XRD analysis showed that there was a certain loss of zeolite crystallinity as a result of the metal loading, which was more pronounced for the Cu-containing samples compared to the Ag-containing samples in agreement with the data reported by other authors.37 Initial experiments were performed with the cheaper Cu-containing materials using E. coli, and the aims were to obtain preliminary data about the activity of the materials and also to compare the activity of Cu-FAZ zeolites to that of the Cu-ZRef sample. Sterile distilled water was used as the suspending medium in all experiments instead of broth culture to avoid introducing complex media and to more closely resemble the conditions in real-life systems.21Fig. 6a shows the relationship between the antibacterial activity of Cu-ZRef and zeolite concentration. Viability in the positive control experiment (E. coli only) remained unchanged throughout the 1 h exposure time. Similar results were obtained for the ZRef sample indicating that the Cu-free zeolite had no antibacterial properties (not shown). A reduction in the number of E. coli cells was measured for all Cu-containing samples and no viable cells were detected after 30 min for the 0.03 g and 0.01 g samples, and after 45 min for the 0.005 g sample. The antibacterial effect of Cu-ZRef may be related to the release of Cu into solution. The amount of copper released after 1 h of exposure was determined as 0.063 ± 0.001 ppm, 0.096 ± 0.003 ppm, and 0.112 ± 0.010 ppm for the 0.005 g, 0.01 g, and 0.03 g of ZRef, respectively. Considering the Cu amount (Table 2) in Cu-ZRef and the zeolite concentrations used, the released Cu constituted 0.05, 0.12 and 0.16% of the Cu content in the Cu-ZRef samples, i.e. the amount of Cu released increased with dilution. The antibacterial tests were performed in distilled water, thus the Cu could not be ion-exchanged into the medium. The release of Cu might be due to zeolite dissolution at the slightly acidic solutions during incubation (pH around 5.5).38 However, more experiments are needed in order to establish the exact relationship between the loaded and released Cu concentrations, in turn the effect of those concentrations on bacterial viability. | ||
| Fig. 6 Antibacterial effect of: (a) 0.005 g, 0.01 g and 0.03 g of Cu-ZRef and (b) 0.03 g of Cu-ZRef, Cu-FAZ4 and Cu-FAZ10 on E. coli over 1 h of exposure. | ||
Next, the antibacterial effect of Cu-ZRef was compared to that of Cu-FAZ samples using 0.03 g of each sample. Negative control experiments of E. coli incubated together with FAZ10 were performed in order to identify any effect of the FAZ samples on recoverable cell numbers. Both negative and positive control experiments showed no decrease in the number of viable cells confirming that the antibacterial effect was due to the presence of copper (Fig. 6b). All three Cu-zeolite samples showed similar antibacterial properties with no E. coli cells recovered after 45 min of exposure.
The antibacterial properties of Ag-zeolites have previously been studied more thoroughly than Cu-zeolites, and Ag has shown higher activity compared to Cu.26 For this reason, the antibacterial activity of the Ag-FAZ samples was further investigated to include other microorganisms and also to establish whether the zeolite particle size influenced the antibacterial efficacy of the Ag-FAZ samples. The change in the viability of E. coli, Ps. aeruginosa and Ent. faecalis during 1 h exposure of the bacterial cultures to 0.01 g of Ag-FAZ zeolites is shown in Fig. 7. The viability of the Gram-negative E. coli and Ps. aeruginosa bacteria was reduced below the detection limits within 15 min of exposure to the two Ag-FAZ samples (Fig.7a and b). The result for E. coli showed that Ag-FAZ samples were more active compared to Cu-FAZ samples as evident from the shorter killing times achieved at lower Ag-FAZ concentrations. The viability of the Gram-positive Ent. faecalis was also reduced below detection limits upon exposure to Ag-FAZ but it took longer, >30 min, to achieve the same antibacterial effect, indicating a higher resistance of this microorganism to the Ag-zeolite treatment (Fig. 7c). The bacterial viability in the presence of FAZ samples remained close to the initial inoculation levels (not shown). The observed differences in the antimicrobial effect of the Ag-FAZ samples on the Gram-negative and Gram-positive bacteria may be due to the much thicker peptidoglycan, in the cell walls of the Gram-positive Ent. faecalis compared to that of E. coli and Ps. aeruginosa.39 The antimicrobial action of silver has been associated with the release of silver ions into the medium containing the bacterial cultures under aerated conditions and the generation of reactive oxygen species.21,40 The release of Ag ions has been found to occur only in the presence of bacteria by some authors.26,40 The Ag concentrations in the supernatants obtained after 1 h exposure of the Ag-FAZ samples to the three different microorganisms were in the range 0.10–0.12 ppm, or about 0.15% of the Ag present in the samples. No trends were evident for the concentrations of released Ag and the presence or absence of a particular type of bacterium for the two Ag-FAZ samples.
 | ||
| Fig. 7 Antibacterial effect of Ag-FAZ4 and Ag-FAZ10 using: (a) E. coli, (b) Ps. aeruginosa and (c) Ent. faecalis over 1 h. | ||
The antimicrobial efficacy of the Ag-FAZ samples following a second exposure to a bacterial inoculum was tested using the Ag-FAZ10 sample and E. coli (Fig. 8). The number of the sequentially added viable cells was again reduced below the detection limits within 15 min, similar to the results shown in Fig. 7a. Thus the Ag-FAZ sample did not lose its activity with time.
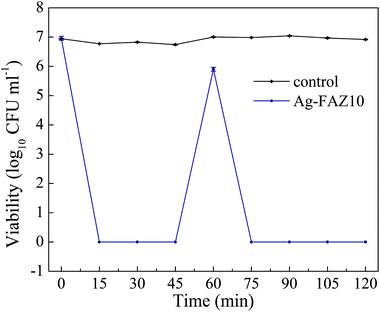 | ||
| Fig. 8 Continued antibacterial effect of Ag-FAZ10 on E. coli after the sequential addition of fresh bacteria. | ||
All 15 min antimicrobial tests showed similar results for the Ag-FAZ4 and the Ag-FAZ10 samples. No viable cells were detected after 15 min in the case of E. coli, and after 45 min in the case of Ent. faecalis independently of the Ag-FAZ sample, Ag-FAZ4 or Ag-FAZ10. To further investigate the antibacterial behaviour of the two samples, 30 s semi-quantitative tests were performed to compare the bactericidal efficacy of the two samples with respect to E. coli and Ent. faecalis. The results shown in Fig. 9 indicated that the Ag-FAZ10 sample reduced the number of viable cells more rapidly than Ag-FAZ4, within 5.2 ± 2.6 min (Ag-FAZ10) compared to 11.7 ± 3.2 min (Ag-FAZ4) for E. coli, and within 38.7 ± 4.7 min (Ag-FAZ10) compared to 50.5 ± 3.5 min (Ag-FAZ4) for Ent. faecalis. The killing times cannot be directly compared to the results obtained for the 15 min tests because of the differing experimental method. The short time period method used only a loopful of the mixture (small sampling volume, high number of cells) that was immediately streaked onto agar without dilution, potentially also carrying some particles with it. The short experiments confirmed that the Gram-positive Ent. faecalis was more resistant to the treatment than E. coli. Also, the micron-sized Ag-FAZ10 sample was found to reduce the number of Gram-positive and Gram-negative cells about 2 and 1.3 times, respectively, faster compared to the nanosized Ag-FAZ4 sample. The differences observed might be due to differences in the release of Ag. The diffusing Ag may become trapped initially within the nanograin boundaries of Ag-FAZ4 thus slowing down the Ag release. Nevertheless, the 15 min quantitative tests indicated that both samples showed similar antibacterial activity.
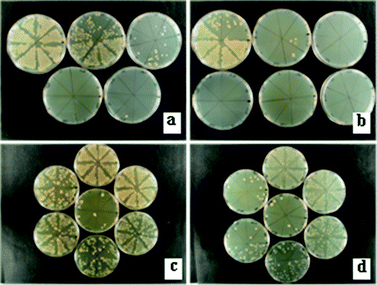 | ||
| Fig. 9 30 s antibacterial tests showing a reduction of growth over time of: (a and b) E. coli on tryptic soya agar plates (0.00–24.00 min) and (c and d) Ent. faecalis on brain–heart infusion agar plates (28.00–56.00 min) in the presence of Ag-FAZ4 (a and c) and Ag-FAZ10 (b and d). The viability of the corresponding cells was reduced over the first: (a) 9.30 min, (b) 3.30 min, (c) 53.0 min and (d) 42.0 min. | ||
4 Conclusions
Micron- and nanosized FAU-type zeolites with a similar degree of crystallinity were prepared from fly ash by alkali fusion in the absence of organic templates. Zeolitisation of the fused alkali ash was activated by the presence of water and involved atomic rearrangements on the mesoscopic scale of the amorphous material yielding a zeolite-like medium-range ordering during ageing. Subsequent hydrothermal treatment resulted in the formation of a zeolite. The size of the zeolite crystals was controlled by the levels of water added to the fused fly ash. Increasing the solid (fused fly ash) to liquid (distilled water) weight ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (sample FAZ10) to 1
10 (sample FAZ10) to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (sample FAZ4) resulted in a decrease in the size of the zeolite crystals. Raman analysis indicated that the structural homogeneity of the precursor zeolite samples was of paramount importance for the homogeneity and characteristics of the zeolite products. The decreased water levels during ageing in the synthesis of FAZ4 resulted in inhomogeneous zeolite nucleation and variation in the size of the nanosized FAU-type primary particles obtained after the hydrothermal treatment. The homogeneity of the FAZ10 precursor improved with ageing, an intermediate-range order resembling that of the FAU-type structure was observed in the Raman spectra, and the crystallized product consisted of uniform micron-sized FAU-type zeolites. Copper and silver were ion-exchanged into the fly ash zeolites and the antibacterial properties of the products were studied. The antimicrobial effect of the Cu-zeolite samples determined using E. coli showed that the Cu-FAZ samples had similar antibacterial properties to a reference Cu-zeolite prepared by conventional hydrothermal treatment. The Ag-FAZ zeolites were more active compared to Cu-FAZ zeolites with respect to E. coli. In addition, they were also effective in killing Ps. aeruginosa and Ent. faecalis, although Ent. faecalis was more resistant, and more time was needed to reduce the viable cells in the presence of Ag-FAZ. The Ag-FAZ samples reduced numbers of microorganisms after sequential challenge without any influence on the killing time. Finally, the semi-quantitative 30 s antibacterial tests indicated that the micron-sized Ag-FAZ10 killed both Gram-positive and Gram-negative microorganisms about 1.3–2 times more rapidly compared to the nanosized Ag-FAZ4. In all cases, the antibacterial effect was associated with the release of Cu or Ag into the solution.
4 (sample FAZ4) resulted in a decrease in the size of the zeolite crystals. Raman analysis indicated that the structural homogeneity of the precursor zeolite samples was of paramount importance for the homogeneity and characteristics of the zeolite products. The decreased water levels during ageing in the synthesis of FAZ4 resulted in inhomogeneous zeolite nucleation and variation in the size of the nanosized FAU-type primary particles obtained after the hydrothermal treatment. The homogeneity of the FAZ10 precursor improved with ageing, an intermediate-range order resembling that of the FAU-type structure was observed in the Raman spectra, and the crystallized product consisted of uniform micron-sized FAU-type zeolites. Copper and silver were ion-exchanged into the fly ash zeolites and the antibacterial properties of the products were studied. The antimicrobial effect of the Cu-zeolite samples determined using E. coli showed that the Cu-FAZ samples had similar antibacterial properties to a reference Cu-zeolite prepared by conventional hydrothermal treatment. The Ag-FAZ zeolites were more active compared to Cu-FAZ zeolites with respect to E. coli. In addition, they were also effective in killing Ps. aeruginosa and Ent. faecalis, although Ent. faecalis was more resistant, and more time was needed to reduce the viable cells in the presence of Ag-FAZ. The Ag-FAZ samples reduced numbers of microorganisms after sequential challenge without any influence on the killing time. Finally, the semi-quantitative 30 s antibacterial tests indicated that the micron-sized Ag-FAZ10 killed both Gram-positive and Gram-negative microorganisms about 1.3–2 times more rapidly compared to the nanosized Ag-FAZ4. In all cases, the antibacterial effect was associated with the release of Cu or Ag into the solution.
The results in this work indicate that nanosized FAU-type zeolites can be prepared from fly ash in the absence of organic templates. It is also shown that Cu- and Ag-fly ash zeolites have a potential for utilization as antimicrobial materials, thus expanding the application areas of fly ash zeolites previously reported.
Acknowledgements
The authors gratefully acknowledge the support of the Engineering and Physical Sciences Research Council Bridging the Gaps programme (grant reference EP/H000291/1). B.M. is indebted to the Deutsche Forschungsgemeinschaft (INST 152/460-1).Notes and references
- L. Tosheva and V. P. Valtchev, Chem. Mater., 2005, 17, 2494 CrossRef CAS.
- S. C. Larsen, J. Phys. Chem. C, 2007, 111, 18464 CAS.
- W. Song, G. Li, V. H. Grassian and S. C. Larsen, Environ. Sci. Technol., 2005, 39, 1214 CrossRef CAS.
- W. Song, R. E. Justice, C. A. Jones, V. H. Grassian and S. C. Larsen, Langmuir, 2004, 20, 4696 CrossRef CAS.
- B. Louis, A. Vicente, C. Fernandez and V. Valtchev, J. Phys. Chem. C, 2011, 115, 18603 CAS.
- S. I. Zones, Microporous Mesoporous Mater., 2011, 144, 1 CrossRef CAS.
- V. P. Valtchev and K. N. Bozhilov, J. Phys. Chem. B, 2004, 108, 15587 CrossRef CAS.
- Y. Huang, K. Wang, D. Dong, D. Li, M. R. Hill, A. J. Hill and H. Wang, Microporous Mesoporous Mater., 2010, 127, 167 CrossRef CAS.
- Y. C. Kim, J. Y. Jeong, J. Y. Hwang, S. D. Kim and W. J. Kim, J. Porous Mater., 2009, 16, 299 CrossRef CAS.
- M. Ahmaruzzaman, Prog. Energy Combust. Sci., 2010, 36, 327 CrossRef CAS.
- X. Querol, N. Moreno, J. C. Umaña, A. Alastuey, E. Hernández, A. López-Soler and F. Plana, Int. J. Coal Geol., 2002, 50, 413 CrossRef CAS.
- X. Querol, J. C. Umaña, F. Plana, A. Alastuey, A. López-Soler, A. Medinaceli, A. Valero, M. J. Domingo and E. Garcia-Rojo, Fuel, 2001, 80, 857 CrossRef CAS.
- M. Norihiro, H. Yamamoto and J. Shibata, Int. J. Miner. Process., 2002, 64, 1 CrossRef.
- H. Tanaka, S. Matsumura and R. Hino, J. Mater. Sci., 2004, 39, 1677 CrossRef CAS.
- T. T. Wałek, F. Saito and Q. Zhang, Fuel, 2008, 87, 3194 CrossRef.
- H. Tanaka and A. Fujii, Adv. Powder Technol., 2009, 20, 473 CrossRef CAS.
- M. Gross-Lorgouilloux, P. Gaullet, M. Soulard, J. Patarin, E. Moleiro and I. Saude, Microporous Mesoporous Mater., 2010, 131, 407 CrossRef CAS.
- N. Shigemoto, H. Hayashi and K. Miyaura, J. Mater. Sci., 1993, 28, 4781 CrossRef CAS.
- H.-L. Chang and W.-H. Shih, Ind. Eng. Chem. Res., 1998, 37, 71 CrossRef CAS.
- A. Molina and C. Poole, Miner. Eng., 2004, 17, 167 CrossRef CAS.
- Y. Inoue, M. Hoshino, H. Takahashi, T. Noguchi, T. Murata, Y. Kanzaki, H. Hamashima and M. Sasatsu, J. Inorg. Biochem., 2002, 92, 37 CrossRef CAS.
- Y. Inoue, M. Kogure, K. Matsumoto, H. Hamashima, M. Tsukada, K. Endo and T. Tanaka, Chem. Pharm. Bull., 2008, 56, 692 CrossRef CAS.
- A. Top and S. Ülkü, Appl. Clay Sci., 2004, 27, 13 CrossRef CAS.
- B. Kwakye-Awuah, C. Williams, M. A. Kenward and I. Radecka, J. Appl. Microbiol., 2008, 104, 1516 CrossRef CAS.
- P. Lalueza, M. Monzón, M. Arruebo and J. Santamaria, Chem. Commun., 2011, 47(2), 680, 10.1039/c0cc03905e.
- R. S. Bedi, R. Cai, C. O'Neill, D. E. Beving, S. Foster, S. Guthrie, W. Chen and Y. Yan, Microporous Mesoporous Mater., 2012, 151, 352 CrossRef CAS.
- P. Payment, M. Waite and A. Dufour, in Assessing microbial safety of drinking water: Improving approaches and methods, ed. A. Dufour, M. Snozzi, W. Koster, J. Bartram, E. Ronchi and L. Fewtrell, IWA Publishing, London, U.K., 2003 Search PubMed.
- K. Norrish and J. T. Hutton, Geochim. Cosmochim. Acta, 1969, 33, 431 CrossRef CAS.
- S. J. Gregg and K. S. W. Sing, in Adsorption, Surface Area and Porosity, Academic Press, London, U.K., 2nd edn. 1982 Search PubMed.
- C. H. Rüscher, Phys. Chem. Miner., 1996, 23, 50 Search PubMed.
- K. J. Kingma and R. J. Hemley, Am. Mineral., 1994, 79, 269 CAS.
- S. Sugai and A. Onodera, Phys. Rev. Lett., 1996, 77, 4210 CrossRef CAS.
- D. W. Matson, S. K. Sharma and J. A. Philpotts, Am. Mineral., 1986, 71, 694 CAS.
- E. Dowty, Phys. Chem. Miner., 1987, 14, 80 CrossRef CAS.
- A. A. Khassin, G. N. Kustova, H. Jobic, T. M. Yurieva, Y. A. Chesalov, G. A. Filonenko, L. M. Plyasova and V. N. Parmon, Phys. Chem. Chem. Phys., 2009, 11, 6090 RSC.
- J. Unvros, S. K. Sharma and F. T. Mackenzie, Am. Mineral., 1991, 76, 641 Search PubMed.
- F. Benaliouche, Y. Boucheffa, P. Ayrault, S. Mignard and P. Magnoux, Microporous Mesoporous Mater., 2008, 80, 80 CrossRef.
- A. Petushkov, J. Freeman and S. C. Larsen, Langmuir, 2010, 26, 6695 CrossRef CAS.
- Q. L. Feng, J. Wu, G. Q. Chen, F. Z. Cui, T. N. Kim and J. O. Kim, J. Biomed. Mater. Res., 2000, 52, 662 CrossRef CAS.
- Y. Matsumura, K. Yoshikata, S. Kunisaki and T. Tsuchido, Appl. Environ. Microbiol., 2003, 69, 4278 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Adsorption isotherm of FA, BJH pore-size distribution plots, XRD patterns of Cu- and Ag-containing fly ash zeolites and Cu-containing zeolite reference sample. See DOI: 10.1039/c2jm33180b |
| This journal is © The Royal Society of Chemistry 2012 |
