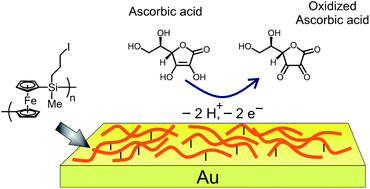
Chemically modified electrodes, decorated with covalently tethered poly(ferrocenylsilane) – PFS chains, are fabricated. Robust, relatively dense redox-active films with a height of around 10 nm are successfully formed by reaction of poly(ferrocenyl(3-iodopropyl)methylsilane) with amine-terminated monolayers on silicon or gold surfaces. The electrochemical properties of the surface-immobilized PFS chains are studied using cyclic voltammetry (CV) and differential pulse voltammetry (DPV), both in aqueous and organic media. Information on the properties of these films as a function of redox state is gained using quantitative adherence measurements between the films and AFM tips. An ascorbic acid electrochemical sensor based on these surface-anchored PFS chains, exhibiting a high sensitivity and stability, was fabricated. The PFS layers described are easily derivatized, thus forming a platform for creating highly tailorable redox-active interfaces.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...