DOI:
10.1039/C2JM30192J
(Paper)
J. Mater. Chem., 2012,
22, 8835-8845
Ordered mesoporous graphitized pyrolytic carbon materials: synthesis, graphitization, and electrochemical properties†
Received
11th January 2012
, Accepted 27th February 2012
First published on 28th February 2012
Abstract
Ordered mesoporous graphitized pyrolytic carbons have been successfully synthesized via direct chemical vapor deposition from methane with mesoporous silicas as the hard templates. The synthesis procedure is quite simple without use of solvent, catalyst or carrying gas, but efficient for producing mesoporous carbon materials. The whole carbon deposition process is comprehensively studied and illustrated, and the mesostructure regularity, pore architecture, and porosity of the resultant carbon materials can be tuned by simply manipulating the deposition time. The morphological, structural, textural and framework properties of the obtained carbon materials are extensively studied, clearly demonstrating the special features including controllable mesostructures, variable mesopore arrangements, large pore volumes (up to 2.3 cm3 g−1), high surface areas (up to 750 m2 g−1) and highly graphitized pore walls with preferred (002) crystal plane orientation. Simple thermal treatment pathways for further promoting the graphitization degree are also proposed. These mesoporous graphitic carbon materials hold promising potential for electrochemical energy storage and conversion applications. They can serve as excellent supports for platinum nanoparticles for oxygen reduction, showing greatly enhanced Pt utilization, activity, methanol tolerance and long-term stability compared to an activated-carbon-supported Pt catalyst. They can be adopted as electrode materials for lithium-ion batteries, showing a high reversible capacity up to ∼340 mAh g−1 and a good cyclic stability. They can also be utilized as electrode materials without the use of any conductive additives for supercapacitors under non-aqueous systems, showing a specific capacitance of ∼40 F g−1 with high Coulombic efficiency and excellent rate performance.
1. Introduction
Porous carbon materials with ordered mesostructures, high porosities and large pore sizes are ubiquitous since the pioneering work of mesoporous carbon CMK-1.1 They possess high potential in many applications including adsorption, separation, sensing, gas storage, energy storage and conversion, etc.2–9 They are further widely adopted as supports for nanoparticles due to their ability to highly disperse guest species,10–12 for example as supports for platinum (Pt) nanoparticles producing essentially important electrocatalysts in fuel cells.10,13–15 As of now, the synthesis of ordered mesoporous carbons from both hard- and soft-templating routes are fairly well developed in terms of controlling the structure, porosity, pore size, morphology and functionality.11,16–22 However, the majority of ordered mesoporous carbons are mainly amorphous in nature, which may be of disadvantage especially in electrochemical applications. As an example, mesoporous amorphous carbon supports show inferior oxidation resistance and low binding interactions toward Pt nanoparticles, leading to severe oxidation and corrosion of the carbon supports, as well as detachment and aggregation of the Pt nanoparticles under fuel cell operations.10,13,23–25 In this regard, graphitic carbons such as nanotubes, graphenes, and porous graphitized carbons are continuously developed and adopted as new supports for Pt nanoparticles with enhanced binding strength and improved long-term stability.25–29 However, most of the above graphitic carbon supports suffer from relatively lower surface areas and smaller pore volumes as compared to those amorphous in nature. Moreover, their surfaces are normally required to be modified for high nanoparticle dispersion. As a result, facile routes for the synthesis of mesoporous carbons combining highly graphitized networks, high surface areas and large pore volumes are in demand.
Conventionally, graphitization is often achieved via very high-temperature thermal treatment (up to 2800 °C). However, it is less successful for the graphitization of mesoporous carbons since the mesostructure can be severely damaged and the porosity can be dramatically damaged, and only a certain degree of graphitization can be fulfilled.30 Local and partial graphitization can be also achieved by in situ catalysis with metal nanoparticles.31–33 The most successful route for graphitizing mesoporous carbons is adopting new precursors, mainly aromatic hydrocarbons.34–37 This route is basically limited to the hard-templating approach due to the templated carbons being mainly composed of nanowires or nanospheres which are easier to graphitize compared to those composed of continuous carbon frameworks synthesized through soft-templating methods.38 With some special aromatic compounds as the precursors (e.g. anthracene, benzene, mesophase pitch), after loading into appropriate silica templates, carbonization and more or less graphitization can be achieved under thermal treatment at relatively low temperatures. However, the synthesis procedure is normally quite complex and time-consuming and the graphitic layers of the carbons are normally perpendicular to the long axis of the nanowires, which is not helpful for enhancing electrical conductivity.34 Alternatively, chemical vapour deposition (CVD) is a fast way for preparing mesoporous graphitic carbons saving solvents and time.39 However, the carbon deposition rate and yield are relatively low due to the low partial pressure of the vaporised carbon precursor (e.g. acetonitrile, benzene) carried by inert gas. It is still a great challenge to synthesize ordered mesoporous carbons with high surface areas, large pore volumes and highly graphitized structures, even the case with the graphitic layers parallel to the mesochannels.
CH4 is commonly adopted for producing pyrolytic carbons in industry, which holds features of simplicity, wide availability, and fast carbon deposition rate with low content of pyrolytic intermediates.40,41 In our previous work, we have shown the applicability to adopt CH4 as a carbon precursor for the synthesis of partially graphitized mesoporous carbons.42 Recently, CH4 has often been adopted for the growth of graphene.43,44
Herein, we demonstrate the controllable and efficient synthesis of ordered mesoporous highly graphitized carbons with high surface areas and large pore volumes by using CH4 as a precursor and various mesoporous silicas as hard templates without use of any solvent, catalyst, or carrying gas. The fast carbon deposition process is studied in detail and the mesostructure and porosity of the resultant carbons are easily tunable. The mesoporous carbons are highly graphitized with the graphitic layers generally parallel to the long axis of the carbon nanowires. Methods for further graphitization promotion are also proposed. These mesoporous graphitic carbons hold promising potential in energy storage and conversion applications. They can serve as excellent supports for Pt nanoparticles, delivering promising oxygen reduction performance with greatly enhanced Pt utilization, activity, methanol tolerance and long-term stability. They can be directly adopted as electrode materials for lithium-ion batteries, showing a high reversible capacity up to ∼340 mAh g−1 and a good cyclic stability. Moreover, they can be also utilized as electrode materials without the use of any conductive additives for supercapacitors, showing a specific capacitance of ∼40 F g−1 with high Coulombic efficiency and excellent rate performance in a non-aqueous system.
2. Experimental section
2.1 Chemicals
The triblock copolymer Pluronic P123 (EO20PO70EO20, Mw = 5800) and Pt(NH3)4Cl2·xH2O were purchased from Sigma-Aldrich. Tetraethoxysilane, n-butanol, aqueous hydrofluoric acid solution (HF, 40 wt%), isopropanol, nafion solution (5 wt%), methanol and sulfuric acid (98 wt%) were purchased from Shanghai Chemical Corporation. CH4 (99.99%) was obtained from Sinopharm. All the chemicals were used as received without further purification. Deionized water was used in all the experiments.
2.2 Synthesis and graphitization of mesoporous carbons
The mesoporous silica templates (e.g. SBA-15 and KIT-6) were synthesized according to the reported procedures.45,46 The ordered mesoporous graphitic carbons (MGCs) were synthesized by a typical CVD process with the mesoporous silicas as the hard templates and CH4 as a precursor.42,47 A typical synthesis procedure was as follows. ∼0.5 g of the mesoporous silica SBA-15 was loosely and evenly placed in a ceramic boat. After loading the boat into a tube furnace and carefully making the whole system air-tight, the furnace was purged with Ar (99.995%, 100 mL min−1) for 1 h at room temperature and then heated to 600 °C with a ramp rate of 2 °C min−1 under Ar (60 mL min−1). At the point of 600 °C, the Ar atmosphere was switched to CH4 (60 mL min−1) and the furnace was further heated to the targeted temperature (700–1000 °C), typically 900 °C, and held isothermal for 20 min–3 h to get different carbon deposition amounts. Then, the CH4 gas was switched back to Ar and the temperature was further kept isothermal for 2 h in order to graphitize. Finally, the furnace was cooled down to <50 °C in a ventilating fume cupboard under an Ar atmosphere. The deposition of carbon along the template layer is generally uniform due to an easy diffusion of the small-size methane gas molecules. The collected black carbon@silica composite (denoted as GC@SBA-15) was immersed and stirred in excess HF solution (10 wt%) overnight to remove the silica component completely. After filtering, washing and drying, carbon products were obtained, which are denoted as MGC-SBA-15-900-t, where MGC stands for mesoporous graphitic carbon, SBA-15 for the template, 900 for the T/°C and t for the CVD time (h).
To further promote the graphitization degree of the MGCs, two methods were adopted. The first one was to further heat the carbon@silica composites (e.g. MGC@SBA-15-900-3h) following the carbon deposition step to 1000 °C for 3 h under Ar, followed by cooling and silica removal. The second method was to further heat the silica-free MGCs obtained as above to 1200 °C and held for 2 h under high vacuum.
2.3 Preparation of carbon supported Pt electrocatalysts
The mesoporous MGCs could be adopted as excellent supports for Pt nanoparticles with high dispersion and strong Pt to graphitic carbon binding. Typically, ∼0.2 g of the mesoporous MGC-SBA-15-900-3h was dispersed by stirring with ∼5 mL of water and ethanol mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume). Then an aqueous solution of Pt(NH3)4Cl2·xH2O with a calculated Pt loading of ∼4 wt% was added, and it was sonicated for 30 min. Then, the solvent was evaporated at 50 °C under continuous stirring. After drying, the composite was heated to 900 °C with a heating rate of 2 °C min−1 and held for 3 h under an Ar atmosphere to carbothermally reduce the Pt precursor to metallic Pt nanoparticles. After cooling, the final electrocatalyst for oxygen reduction was obtained. The final Pt content was determined to be ∼4.7 wt%. A commercial activated carbon supported Pt electrocatalyst with ∼5.6 wt% of Pt, highly dispersive in nature, was used as a counterpart for comparison. Its structure and porosity, as well as the size and distribution of the Pt nanoparticles, were well characterized and can be found in the ESI.†
1 by volume). Then an aqueous solution of Pt(NH3)4Cl2·xH2O with a calculated Pt loading of ∼4 wt% was added, and it was sonicated for 30 min. Then, the solvent was evaporated at 50 °C under continuous stirring. After drying, the composite was heated to 900 °C with a heating rate of 2 °C min−1 and held for 3 h under an Ar atmosphere to carbothermally reduce the Pt precursor to metallic Pt nanoparticles. After cooling, the final electrocatalyst for oxygen reduction was obtained. The final Pt content was determined to be ∼4.7 wt%. A commercial activated carbon supported Pt electrocatalyst with ∼5.6 wt% of Pt, highly dispersive in nature, was used as a counterpart for comparison. Its structure and porosity, as well as the size and distribution of the Pt nanoparticles, were well characterized and can be found in the ESI.†
2.4 Measurements and characterization
Small-angle X-ray scattering (SAXS) patterns were recorded on a Nanostar U small-angle X-ray scattering system (Germany) using Cu-Kα radiation (40 kV, 35 mA) with a recording time of 15 min for each sample. X-ray diffraction (XRD) patterns were recorded with a Bruker D4 X-ray diffractometer (Germany) with Ni-filtered Cu-Kα radiation (40 kV, 40 mA). Nitrogen sorption isotherms were measured at −196 °C with a Micromeritics Tirstar 3020 or an ASAP 2020 analyzer. Before the measurements, the samples were degassed under vacuum at 180 °C for at least 8 h. The surface area (SBET) was calculated with the Barrett–Emmett–Teller (BET) method by using the adsorption data at a relative pressure range of 0.04–0.25. The pore size distribution (PSD) curve was retrieved with the Barrett–Joyner–Halanda (BJH) model by using the adsorption branch. The total pore volume (Vt) was obtained based on the N2 adsorbed amount at a relative pressure of ∼0.995. Scanning electron microscopy (SEM) images were taken by a Hitachi S4800 or a JEOL 6300F scanning electron microscope (Japan) operated at an accelerated voltage of 1 or 20 kV. Transmission electron microscopy (TEM) experiments were conducted on a JEOL 2100F (Japan) or a Phillips CM20 (Netherlands) microscope both operated at 200 kV. The samples for TEM measurements were suspended in ethanol and dropped onto holey carbon films supported on Cu grids. Thermogravimetric (TG) analyses were conducted on a Mettler Toledo TGA/SDTA851 analyzer (Switzerland) from 25 to 900 °C (5 °C min−1) under flowing O2 (99.95%, 20 mL min−1). Raman spectra were recorded with a Dilor LabRam-1B Raman spectroscope by using an exciting wavelength of 632.8 nm from a He–Ne laser.
2.5 Electrochemical tests
2.5.1 Oxygen reduction reaction (ORR).
Tests such as cyclic voltammetry (CV) measurements were conducted on a CHI 606B electrochemical analyzer system (Shanghai CHI Instruments Co.) under ambient conditions. A three-electrode glass cell was used, in which a Pt wire was used as the counter electrode and a saturated calomel electrode (SCE, 0.2415 V vs. the normal hydrogen electrode, NHE) as the reference electrode. The working electrode was made from the carbon-supported Pt catalysts and nafion as a binder. Typically, ∼2.2 mg of the typical MGC (MGC-SBA-15-900-3h) supported Pt catalyst dispersed in ∼100 μL of isopropanol was mixed with ∼100 μL of a 0.5 wt% nafion solution (in isopropanol) and ultra-sonicated at room temperature for 30 min. ∼5 μL of the above-prepared catalyst ink was injected onto a polished glassy carbon electrode and dried under ambient conditions for 15 min before electrochemical measurement. A 0.5 M H2SO4 solution in the absence or presence of 0.5 M CH3OH was adopted as the electrolyte. CV measurements were conducted under N2- or O2-saturated conditions over a potential range of −0.2–1.2 V (vs SCE) with a scan rate of 10 mV s−1. Potential cycling was conducted for ∼5 min before stable curves were recorded. The rotating disk electrode (RDE) technique was employed to study the ORR activity and kinetics with a rotating speed of 400–1600 rpm and a typical scan rate of 10 mV s−1.
2.5.2 Lithium-ion battery.
The tests such as charging/discharging profiles were performed with a CR2016-type coin cell. Metallic lithium was used as the negative electrode. To prepare the working electrode, 85 wt% of the mesoporous MGC sample obtained by the graphitization promotion at 1200 °C for 2 h under vacuum, 10 wt% of carbon black and polyvinylidene fluoride dissolved in N-methylpyrrolidinone were well mixed and the slurries were coated onto a Cu foil. Then, the electrode was dried at ∼80 °C for 10 min to evaporate the solvent before pressing. The electrode was cut into sheets of ∼1 cm2 in area, dried at 100 °C under vacuum for 24 h, and then weighed before assembly. The typical mass load on the electrode of the active material was ∼5 mg cm−2. The cell assembly was operated in a glove box (model 100G, MBraun, Germany) filled with Ar. The electrolyte solution was 1 M LiPF6/ethylene carbonate/diethyl carbonate/ethyl methyl carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume). The cell was assembled from the as-prepared working electrode, lithium negative electrode, and separators made of a Celgard 2300 film. Charge/discharge experiments were conducted at a rate of 0.1 C between 0.01 and 3.0 V using a LAND CT2001A Battery Cycler (Wuhan, China). Lithium insertion into the working electrode was referred to as discharge and extraction as charge. CV measurements were carried out on a CHI Instruments electrochemical workstation (model 600A) with a scan rate of 1 mV s−1. The metallic lithium was used as counter and reference electrode.
1 by volume). The cell was assembled from the as-prepared working electrode, lithium negative electrode, and separators made of a Celgard 2300 film. Charge/discharge experiments were conducted at a rate of 0.1 C between 0.01 and 3.0 V using a LAND CT2001A Battery Cycler (Wuhan, China). Lithium insertion into the working electrode was referred to as discharge and extraction as charge. CV measurements were carried out on a CHI Instruments electrochemical workstation (model 600A) with a scan rate of 1 mV s−1. The metallic lithium was used as counter and reference electrode.
2.5.3 Supercapacitor.
The mesoporous MGC obtained through the graphitization step at 1000 °C was adopted as an electrode material for a supercapacitor under a non-aqueous system. The working electrode was prepared as follows: the active material and polytetrafluoroethylene (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in weight ratio) were well mixed without the use of any conductive additive such as acetylene black in order to make the high conductivity of the MGCs stand out. The slurries were coated on an aluminum foil which was employed as current collectors for both positive and negative electrodes. After coating, the electrode was dried at 80 °C for 10 min to remove the solvent before pressing. The electrode was cut into disks of ∼1.2 cm in diameter, vacuum-dried at 100 °C for 24 h, and weighed. The typical active mass loading on the electrode was ∼5 mg cm−2. The electrolyte was 1.0 M (C2H5)4NBF4 in propylene carbonate (Honeywell Corp). CV curves and galvanostatic charge/discharge profiles were measured on a CHI 760 electrochemical working station under ambient conditions.
10 in weight ratio) were well mixed without the use of any conductive additive such as acetylene black in order to make the high conductivity of the MGCs stand out. The slurries were coated on an aluminum foil which was employed as current collectors for both positive and negative electrodes. After coating, the electrode was dried at 80 °C for 10 min to remove the solvent before pressing. The electrode was cut into disks of ∼1.2 cm in diameter, vacuum-dried at 100 °C for 24 h, and weighed. The typical active mass loading on the electrode was ∼5 mg cm−2. The electrolyte was 1.0 M (C2H5)4NBF4 in propylene carbonate (Honeywell Corp). CV curves and galvanostatic charge/discharge profiles were measured on a CHI 760 electrochemical working station under ambient conditions.
3. Results and discussion
3.1 Control of carbon deposition
The most appropriate temperature for fast carbon deposition from CH4 is found to be 900 °C, while it requires a long time to obtain a considerable carbon amount at ≤800 °C, and substantial extra carbon deposition on the external surface of the mesoporous silica template and accumulation of intermediates can occur at ≥1000 °C.42 The amount deposited at 900 °C can be easily manipulated by adjusting the CVD time, thus efficiently controlling the mesostructure and porosity of the resultant carbon materials. With a deposition time of 0.5–1.5 h (Fig. 1a–c, and inset), gentle increases of deposited carbon amounts from ∼0.25 to ∼0.35 g g−1 (carbon/SiO2) are observed. It further increases to ∼0.6 g g−1 at a prolonged deposition time of 2.0 h (Fig. 1d) and rises rapidly to ∼1.75 g g−1 at a further extended time of 3 h (Fig. 1e). Such a gradually increased deposition rate is related to the pre-deposited carbon being able to remarkably accelerate subsequent deposition.40 At a time of 3 h, the pore volume (∼1.0 cm3 g−1) of the mesoporous silica SBA-15 template is almost fully occupied because the deposited carbon amount (1.75 g g−1) possesses a volume of approximately ∼1.0 cm3 g−1. This full carbon loading is illustrated by the fact that the GC@SBA-15-900-3h composite shows quite a limited N2 adsorbed amount (Fig. 2A, f). As a result, a deposition time longer than 3 h leads to substantial extra carbon deposition around the external surface of the silica template reducing porosity. As shown above, the synthesis procedure is quite fast and efficient with large quantities of carbon obtained in a short time without any wet impregnation step and carrying gas, which is advantageous (saving time, solvent and energy) over the conventional templating synthesis of mesoporous carbon materials. Under optimal conditions,48 ordered MGCs can be quickly prepared within 3 h. The synthesis can be also scaled up to obtain ∼20 g of carbon in a single pot in our lab. Moreover, the flowing rate of CH4 can be well controlled with a high carbon conversion.
 |
| | Fig. 1 TG curves under flowing O2 of the mesoporous GC@SBA-15 composites obtained at 900 °C with a CVD time of 0.5 (a), 1.0 (b), 1.5 (c), 2.0 (d), and 3.0 h (e). The inset shows the corresponding time-dependant curve for the deposited carbon amount. | |
 |
| | Fig. 2 N2 sorption isotherms (A) and the corresponding PSD curves derived from the adsorption (B) and desorption branches (C) of the mesoporous GC@SBA-15 composites obtained at 900 °C with a CVD time of 0 (a), 0.5 (b), 1.0 (c), 1.5 (d), 2.0 (e) and 3.0 h (f). | |
3.2 Illustration of the carbon deposition process
In order to understand the carbon deposition process and finely control the mesostructure and porosity of the resultant MGC materials, the mesoporous GC@SBA-15 intermediates were carefully studied (Fig. 2) and a schematic illustration of the whole carbon deposition process was proposed (Fig. 3). N2 sorption isotherms (Fig. 2A) of the GC@SBA-15 composites obtained at different CVD durations show gradual decreases of the N2 adsorbed amounts and thus of the surface areas and pore volumes (Table 1). Interestingly, with the increase of the deposition amounts, the relative pressures of N2 condensation shift to lower values (Fig. 2A), indicating decreases in pore sizes. Within a short deposition time of 0.5 h, the pore size derived from the adsorption branch of the GC@SBA-15 composite decreases from ∼9.2 to ∼7.6 nm (Fig. 2B, a, b), and the pore window size derived from the desorption branch also decreases (Fig. 2C, a, b), indicating that a thin layer of carbon is probably deposited (Fig. 3a). With the deposition time prolonged to 1.0–2.0 h, the pore sizes derived from the adsorption branches of the corresponding GC@SBA-15 composites almost remain changeless (Fig. 2B, c–e and Table 1), but the pore window sizes derived from the desorption branches gradually decrease (Fig. 2C, c–e and Table 1), suggesting that more and more carbon is deposited near the pore openings (Fig. 3b). This is because there is diffusion resistance for the carbon species deep into the pore channels, resulting in non-uniform carbon deposition along the mesochannels and thus ink-bottle-like mesopores in the composites (Fig. 3b). Nevertheless, with the deposition time extended to 3.0 h, a limited N2 adsorbed amount (Fig. 2A, f) is observed without any peaks in the PSD curves (Fig. 2B, f and 2C, f). It implies that the pore voids of the silica template are almost fully filled, but some buried pores (especially micropores) probably exist due to the diffusion limitation deep into the mesochannels (Fig. 3c). Meanwhile, during the CVD process, more or less carbon nanoflakes and/or nanohorns (see below) are grown on the external surface of the silica template, which is probably due to the epitaxial carbon deposition/growth from the carbon nanorods deposited within the complementary pores of the silica template (Fig. 3).
 |
| | Fig. 3 A schematic illustration of the carbon deposition process within the mesoporous silica SBA-15 template at 900 °C at a CVD time of 0.5 (a), 0.5–2.0 (b) and 2.0–3.0 h (c). | |
Table 1 The textural properties of the mesoporous GC@SBA-15 composites and the resultant MGC materials obtained at 900 °C with various CVD durations
| Sample name |
CVD time (h) |
S
BET (m2 g−1) |
V
t (cm3 g−1) |
D
ads
b (nm) |
D
des
c (nm) |
|
The mesoporous silica SBA-15 template was calcined under Ar at the same CVD temperature for 2 h without carbon deposition.
The pore size derived from adsorption branch.
The pore size derived from the desorption branch.
|
| SBA-15a |
0 |
411 |
0.92 |
9.2 |
6.2 |
| GC@SBA-15-900-0.5h |
0.5 |
366 |
0.67 |
7.6 |
6.1 |
| MGC-SBA-15-900-0.5h |
200 |
1.00 |
4–11 |
— |
| GC@SBA-15-900-1h |
1.0 |
399 |
0.60 |
7.1 |
6.0 |
| MGC-SBA-15-900-1h |
577 |
1.80 |
3.6–5.4, 18 |
— |
| GC@SBA-15-900-1.5h |
1.5 |
332 |
0.50 |
7.0 |
5.4 |
| MGC-SBA-15-900-1.5h |
623 |
2.30 |
3.6–5.4, 19 |
— |
| GC@SBA-15-900-2h |
2.0 |
299 |
0.39 |
7.0 |
3.7 |
| MGC-SBA-15-900-2h |
529 |
1.3 |
3.6–5.4, 18 |
— |
| GC@SBA-15-900-3h |
3.0 |
10 |
0.04 |
— |
— |
| MGC-SBA-15-900-3h |
273 |
0.49 |
3.7 |
— |
3.3 Mesostructure, porosity and graphitic nature
The mesostructural regularity of the mesoporous MGCs obtained at different CVD durations can be clearly elucidated by the SAXS patterns (Fig. 4A). Only two wide and weak scattering peaks are observed for the sample obtained at a short deposition time of 0.5 h (Fig. 4A, a), attributed to a poor mesostructural regularity, which is resulted from a low carbon loading in the silica template leading to structure collapse after the template removal. Nevertheless, the macroscopic wheat-like morphology is well preserved (Fig. 5b). However, the carbon ‘wheat’ is composed of numerous connected carbon ‘nanoislands’ (∼30 nm in size) other than continuous rods as the case for the silica template (Fig. 5a). There are many large mesopore voids between adjacent carbon ‘nanoislands’, further suggesting that the deposited carbon network is not enough to support the whole ordered mesostructure, which is mostly collapsed with the formation of large mesopores by coalescence after template removal.49 The N2 sorption result (Fig. 4B, a) shows that the carbon sample possesses a wide PSD at 3.5–20 nm with a relatively low surface area (Table 1). The TEM image (Fig. 6a) shows a low mesostructure regularity. With the deposition time extended to 1.0–2.0 h, the SAXS patterns of the resultant MGC samples show four to five scattering peaks with gradually increased resolution and intensity (Fig. 4A, b–d), indicating that the mesostructural regularity is greatly enhanced due to the increased carbon loadings within the silica template. Interestingly, the intensities of the 11 diffractions are relatively stronger and well-resolved compared to those of the 10 diffractions, implying that the mesostructure in these MGC samples is probably similar to the CMK-5-type architecture (see below).10,50 Besides, their N2 sorption results reveal relatively wide PSDs at 3.6–5.4 nm (Fig. 4B, b–d), a typical feature of CMK-5-type carbon materials. However, at a deposition time of 1.0–2.0 h, N2 sorption results show that a large proportion of the pore voids of the template are still unfilled (Fig. 2A, d–e). As a result, upon the silica template removal, the unoccupied pore voids are collapsed and merged into larger ones. Therefore, there is another PSD centred at ∼18 nm in the resultant MGC samples (Fig. 4B, b–d). Thus, an important feature is that these MGCs possess very high pore volumes along with high surface areas, achieving up to ∼2.3 cm3 g−1 and 623 m2 g−1 for the mesoporous MGC-SBA-15-900-1.5h obtained at a CVD time of 1.5 h (Fig. 4B, c and Table 1). SEM images (Fig. 5c–e) also show a great number of large mesopore voids (10–20 nm) along with the ordered mesopore arrays and disappearance of the ‘nanoisland’ morphology due to the enhanced structure integrity. TEM images (Fig. 6b–d) of the MGCs obtained at a time of 1.0–2.0 h further show ordered hexagonal mesostructure in large domains. Moreover, carbon nanoflakes and/or nanohorns are also observed on the external surface of the carbon pore walls. Under careful observations, carbon nanopipe arrays can be observed (Fig. 6d). It is obvious that the carbon nanowires are hollow-like (inset in Fig. 6d), but the inner mesopores are not uniform but look like connected small cavities due to the uneven deposition of carbon in the silica template (Fig. 3b). Finally, as the deposition time extends to 3 h, the SAXS pattern (Fig. 4A, e) of the resultant MGC shows five well-resolved peaks assigned to the 10, 11, 20, 21, 30 scatterings of a highly ordered hexagonal mesostructure (space group, p6m). Under this condition, the silica pore space is almost fully filled and thus the resultant MGC is similar to CMK-3.51 As a result, the intensity of the 10 scattering in the SAXS pattern is stronger than that of the 11 one. Moreover, N2 sorption results reveal a narrow PSD at ∼3.7 nm (Fig. 4B, e). The surface area (∼273 m2 g−1) and pore volume (∼0.49 cm3 g−1) are lower compared with those of CMK-3 due to the high graphitization degree with limited micropores. The SEM image shows no large mesopore voids along the carbon nanorods (Fig. 5f). The TEM image (Fig. 6e) presents highly ordered strip-like mesopore arrays. Interestingly, more and larger carbon nanoflakes located on the external surface are observed, which are connected by the mesopore carbon walls. The high-resolution TEM (HRTEM) image (Fig. 6f) reveals that the MGC sample has clear crystalline lattices with a d-spacing of ∼0.34 nm, assigned to the (002) plane of graphite, indicating a highly graphitic structure. Moreover, the direction of the (002) crystalline lattices is basically along the long axis of the carbon nanowires or the short nanorods connecting adjacent nanowires, which is beneficial for enhancing electrical conductivity.34 The corresponding selected area electron diffraction (SAED) patterns (insets in Fig. 6a, e) of the MGCs show three bright diffraction rings assigned to the (002), (101) and (112) planes of graphite.
 |
| | Fig. 4 SAXS patterns (A) and N2 sorption isotherms with an inset of the corresponding PSD curves (B) of the mesoporous graphitic carbons obtained at 900 °C with a CVD time of 0.5 (a), 1.0 (b), 1.5 (c), 2.0 (d) and 3.0 h (e). | |
 |
| | Fig. 5 SEM images of the mesoporous silica SBA-15 template (a) and the mesoporous graphitic carbons obtained at 900 °C with a CVD deposition time of 0.5 (b), 1.0 (c), 1.5 (d), 2.0 (e) and 3.0 h (f). | |
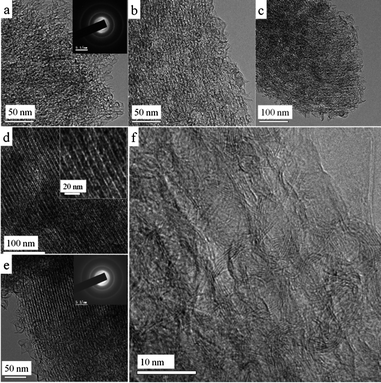 |
| | Fig. 6 TEM (a–e), HRTEM (f) images and SAED patterns (insets in a, e) of the mesoporous graphitic carbons obtained at 900 °C with a CVD carbon deposition time of 0.5 (a), 1.0 (b), 1.5 (c), 2.0 (d) and 3.0 h (e, f). Inset in d is a magnified TEM image showing the nanopipe-like arrays. | |
The graphitic nature of the mesoporous MGC samples is further illustrated by thermogravimetric (TG) analyses, wide-angle XRD patterns and Raman spectra. The TG curve under flowing O2 of the typical mesoporous MGC-SBA-15-900-3h shows a high oxidation resistance, only starting to be oxidized until ∼540 °C and completely combusted at ∼670 °C (Fig. S1a†), higher than those of conventional mesoporous carbons with amorphous frameworks (e.g. CMK-3 and FDU-15). The wide-angle XRD pattern shows stronger and narrower (002) and (101) diffractions (Fig. 7A, a) compared to the amorphous carbon (Fig. 7A, c), indicating a high graphitization degree. The Raman spectrum shows narrow, strong and not overlapped D and G bands (Fig. 7B, a), similar to those of graphitic carbon nanohorns,52 while the amorphous mesoporous carbon shows weak, wide and severely overlapped G and D bands (Fig. 7B, c).
 |
| | Fig. 7 Wide-angle XRD patterns (A) and Raman spectra (B) of the mesoporous MGC-SBA-15-900-3h (a), the resultant mesoporous graphitic carbon (b) obtained by thermally treating the mesoporous GC@SBA-15-900-3h composite at 1000 °C for 3 h under Ar followed by the silica template removal, and the typical mesoporous amorphous carbon FDU-15 (c) obtained at 900 °C. | |
 |
| | Fig. 8 TEM (a, b, d) and HRTEM (c, e, f) images and SAED pattern (inset in f) of the resultant mesoporous graphitic carbon obtained by a thermal treatment of the mesoporous GC@SBA-15-900-3h composite at 1000 °C for 3 h under Ar followed by the silica template removal. | |
Following the carbon deposition step, we avoided a temperature of higher than 1000 °C to improve graphitization due to the possible formation of silicon carbide (Fig. S1b†). To further enhance the graphitization degree, the above-obtained silica-free MGC sample was heated to 1200 °C and held for 2 h under high vacuum. The SAXS pattern (Fig. S6a†) shows that the ordered mesostructure is well retained. Interestingly, the 11 scattering is intensified and narrowed, suggesting the retainment of CMK-5-type mesostructure. The wide-angle XRD pattern (inset in Fig. S6a†) shows sharpened (002) and (101) diffractions. N2 sorption isotherms (Fig. S6b†) present a significantly changed shape with a relatively broad PSD at 3.7–5.0 nm and another wide mesopore distribution at ∼20 nm, indicating that the mesostructural regularity is deteriorated to some extent. The surface area and pore volume are calculated to be ∼586 m2 g−1 and 1.27 cm3 g−1, respectively. The SEM images (Fig. S7†) show that the wheat-like morphology is preserved with the observation of a large number of carbon nanoflakes and the tube-like nanowires. The low-magnification TEM image (Fig. 9a) shows plenty of large mesopore voids across the whole domain. The mesostructural regularity is deteriorated to some extent due to the extensive carbon rearrangement and crystallization (Fig. 9b). CMK-5-like mesostructure is directly observed (Fig. 9c, d). HRTEM images (Fig. 9e, f) clearly reveal the highly crystalline nature of the pore walls with the (002) planes generally parallel to the long axis. The SAED pattern (inset in Fig. 9e) shows brightened diffraction rings presenting a more asymmetric (002) diffraction. The outer layers of the carbon are highly graphitized (Fig. S8†). The carbon nanoflakes and/or nanohorns located on the external surface are also further crystallized with the observation of many graphene-like structures (Fig. 9g).
 |
| | Fig. 9 TEM (a–d, g) and HRTEM (e, f) images and SAED pattern (inset e) of the resultant mesoporous graphitic carbon obtained by the graphitization promoting step at 1200 °C for 2 h under vacuum. | |
3.5 Versatility of the synthesis
The CH4 CVD synthesis is suitable for developing a series of MGCs with variable mesostructures. For example, by using a mesoporous silica KIT-6 as a hard template, with a CVD time of 1.0–3.0 h at 900 °C, disordered or ordered MGCs with Ia3d symmetry can be obtained (Fig. S9, S10†). It is found that the cubic mesostructured carbons possess relatively lower graphitization degree compared with the hexagonal counterparts (data not shown), which is probably due to the easier graphitization of carbon along two-dimensional domain. Nevertheless, with a promotion of graphitization at 1200 °C under vacuum, ordered mesoporous carbon with Ia3d symmetry and highly graphitized frameworks is obtainable, which is not reported previously. The SAXS pattern (inset in Fig. 10a) shows a well retained cubic Ia3d mesostructure. SEM images (Fig. 10a, b) of the carbon product show the same morphology as the silica template and ordered mesopore arrays. TEM images (Fig. 10c, d) show typical planes of Ia3d symmetry. The HRTEM image (inset in Fig. 10d) clearly shows (002) crystal-plane lattices along with the carbon pore walls.
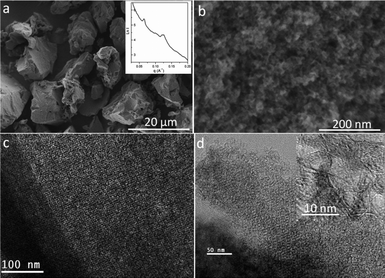 |
| | Fig. 10 SAXS pattern (inset in a), SEM (a, b), TEM (c, d) and HRTEM (inset in d) images of the resultant mesoporous graphitic carbon obtained by heating the mesoporous MGC-KIT-6-900-3h at 1200 °C for 2 h under vacuum. | |
3.6 Evaluation of electrochemical performance
3.6.1 Supporting Pt nanoparticles for ORR.
Mesoporous carbon materials are widely adopted as supports for Pt nanoparticles producing essentially important electrocatalysts, but the amorphous nature is a disadvantage for practical requirements, especially for long-term stability. The mesoporous MGCs can be adopted as excellent supports for Pt nanoparticles as fuel-cell cathodes with promising performance toward the ORR. As a demonstration, the ordered mesoporous MGC-SBA-15-900-3h is loaded with ∼4.7 wt% of Pt nanoparticles through a simple impregnation step followed by carbothermal reduction at 900 °C (see experimental section for details). The ordered mesostructure is well preserved with enhanced surface area and pore volume (Fig. S11†). The wheat-like morphology with plenty of carbon nanoflakes on the external surface is also observed (Fig. S12a, b†). The Pt nanoparticles are homogeneously dispersed (Fig. S12c, d†), showing a particle size range of 3–9 nm with a small fraction of larger sizes of ∼25 nm (Fig. S12e, f†), indicating a good dispersion without sintering even under a high-temperature treatment at 900 °C due to the strong binding between graphitic carbon and Pt.25 Commercial activated carbon supported Pt nanoparticles (∼5.6 wt%, 2–4 nm) (Fig. S13†) were used as the counterpart catalyst for comparison.
The CV curve (Fig. 11A, b) recorded under O2-saturated H2SO4 electrolyte (0.5 M) presents a much higher current density at −0.2–0.5 V with a well-defined ORR signal compared with that recorded under N2-saturated electrolyte (Fig. 11A, a), evidence of high ORR activity. With a similar Pt load (∼13.5 μg cm−2) on the electrode, the MGC supported Pt catalyst (Fig. 11B, a) shows a much higher mass activity than that of the commercial one (Fig. 11B, b) although the Pt nanoparticle sizes are much larger for the former. The limiting current density is up to ∼160 mA mg−1 Pt, much higher than that (∼80 mA mg−1) of the counterpart catalyst. Such better performance is contributed to by three factors. Firstly, the highly graphitized carbon support has lower electrical resistance providing sufficient and fast electron pathways. Secondly, the interaction between Pt and graphitic carbon is strengthened to a large extent compared to that of the amorphous carbon.54 Thirdly, the mesostructure with regular and large mesopore sizes can provide a large interface and also facilitate fast electrolyte infiltration and mass transportation during the electrochemical reaction. As a result, much enhanced utilization and activity of the Pt nanoparticles in the mesoporous MGC support are achieved. The RDE technique (400–1600 rpm) was used to study the kinetics of the ORR on the MGC supported Pt catalyst (Fig. 11C). The corresponding Koutecky-Levich plots at 0.10–0.30 V (Fig. 11D) exhibit a good linearity with the slopes remaining generally constant. The electron transfer number is calculated to be ∼3.99 according to the Koutecky-Levich equation (see the ESI† for details), indicating a four-electron transfer mechanism of the ORR over the MGC supported Pt catalyst. The limiting kinetic current at 0.1 V is calculated to be ∼4.0 mA cm−2. Furthermore, the MGC supported Pt catalyst shows a methanol tolerance behavior (Fig. S14A†) compared to the commercial catalyst, indicated by the absence of a methanol oxidation signal in the CV curve (inset in Fig. S14A†). This is due to the fact that the carbothermally reduced Pt nanoparticles in the MGC support are more or less embedded, which can limit the infiltration of methanol to the Pt surface, while the Pt nanoparticles in the commercial catalyst are loosely dispersed and easily accessible for methanol. As a result, due to the parasitic methanol oxidation, dramatic degradation of the ORR performance of the activated-carbon-supported Pt catalyst after potential cycling occurs (Fig. S14B, b†).14,55 On the contrary, the ORR activity and cyclic stability of the MGC supported Pt catalyst in the presence of methanol are improved (Fig. S14B, a†).
 |
| | Fig. 11 CV curves (A) recorded under N2- (a) and O2-saturated (b) H2SO4 electrolyte (0.5 M), polarization curve (1600 rpm) in mass activity (B, a), polarization curves (400–1600 rpm) in specific activity (C) recorded under O2-purging conditions, and the corresponding Koutecky-Levich plots at various potentials (D) of the mesoporous MGC-SBA-15-900-3h supported Pt catalyst with a Pt content of ∼4.7 wt%. Curve (b) in (B) is the polarization curve (1600 rpm) of the commercial activated carbon supported catalyst with a Pt content of ∼5.6 wt%. The Pt load of the catalysts on the electrode is ∼13.5 μg cm−2. | |
3.6.2 Lithium-ion batteries.
Porous carbon materials are widely used as electrode materials for lithium-ion batteries due to the high specific capacity and negative redox potentials. The capability of insertion and extraction of lithium ions on carbon materials is greatly dependant on their crystallinity, morphology and structure features on the nanoscale. Here, the mesoporous MGC obtained through a graphitization promoting step at 1200 °C under vacuum was evaluated. The CV curve (Fig. 12a) shows that there is an irreversible lithium ion intercalation process (∼0.65 V) with a total capacity of ∼750 mAh g−1 in the first charging cycle. The reversible capacity is found to be ∼340 mAh g−1. The shape of the CV curve is quite similar to that of a highly graphitized carbon onion obtained at 2800 °C with a capacity of ∼200 mAh g−1.56 An obvious peak located at ∼0.2 V standing for lithium extraction from carbon is observed, which is intrinsically different from that of mesoporous amorphous carbon electrode in which the lithium extraction covers a wide potential range,4 suggesting a high graphitic nature of the MGC sample. The cyclic stability is good with a capacity of ∼250 mAh g−1 retained after ten charging/discharging cycles. The good performance is due to the combination of high mesoporosity with regular mesostructure in the nanoscale and high electrical conductivity of the highly graphitized carbon walls.
 |
| | Fig. 12 CV curves (left) and charging/discharging cycles (right) at a rate of 0.1 C of the mesoporous graphitic carbon sample obtained though the graphitization promoting step at 1200 °C for 2 h under high vacuum. | |
3.6.3 Supercapacitors.
Mesoporous carbon materials are widely adopted as electrode materials for supercapacitors.57–59 Here, the MGC sample obtained by heating the GC@SBA-15-900-3h composite at 1000 °C followed by the silica template removal was tested as an electrode material under a non-aqueous system (a 1.0 M (C2H5)4NBF4 in propylene carbonate solution as the electrolyte), due to their hydrophobic nature with a low content of oxygen (∼3 wt%). In order for the high electrical conductivity of the MGC sample arising from the graphitic nature with preferred (002) orientation to stand out, we prepared the working electrode without the use of any conductive additives (see details in experimental section). CV curves (Fig. 13a) show similar rectangular shapes even as the scan rate is increased from 5 to 200 mV s−1, indicating a typical double-layer capacitance behavior with an excellent performance at high scan rates. The charge/discharge profiles collected from 0.5 to 20 A g−1 present isosceles triangular shapes (Fig. 13b), suggesting an excellent Coulombic efficiency and ideal capacitance performance. No sudden potential drop is observed even at a high current density of 20 A g−1 (data not shown), revealing that the MGC-based electrode has a low equivalent series resistance. The specific capacitance is calculated to be ∼40 F g−1 at a current density of 0.5 A g−1 and it is well retained upon a significant increase of the current density to 5 A g−1 (Fig. 13c). About 86 and 70% of the capacitance is retained even though the current density is enhanced to 10 and 20 A g−1, suggesting an excellent rate performance. The reasons for the high performance of the MGC-based supercapacitor are mainly two-fold. First, the highly graphitic and connected carbon networks can facilitate fast electron transport even in the absence of any conductive additives. Second, the high surface area and pore volume can promote the infiltration of the electrolyte (C2H5)4NBF4 (in propylene carbonate solution) and shorten the distance of electrolyte ion diffusion.
 |
| | Fig. 13 CV curves (a) at various scan rates (5–200 mV s−1), charge/discharge profiles (b) at different current densities (0.5–5.0 A g−1) and the dependence (c) of the specific capacitance on increasing current density of the mesoporous graphitic carbon obtained by heating the mesoporous MGC@SBA-15-900-3h composite at 1000 °C for 2 h followed by the silica template removal. | |
4. Conclusions
Mesoporous pyrolytic carbon materials with well controllable mesostructures (disordered, ordered p6m, Ia3d, etc.), high surface areas (up to ∼750 m2 g−1), large pore volumes (up to ∼2.3 cm3 g−1) and highly graphitized carbon pore walls have been synthesized with a facile CVD method by using CH4 as a precursor. The synthesis procedure is quite simple through direct pyrolysis of CH4 at 900 °C without the use of any solvent, catalyst or carrying gas, but very efficient with a fast carbon deposition rate and a high carbon yield, providing a time- and energy-saving and scalable synthetic route for mesoporous graphitic carbon, advantageous over conventional synthesis of mesoporous carbons. With a single mesoporous silica (i.e. SBA-15) as the hard template, the mesostructure (disordered, CMK-3- and CMK-5-type) and porosity of the resultant MGCs can be conveniently controlled by tuning the carbon deposition time (0.5–3 h). The whole deposition process and the properties of the resulted carbon materials at each stage are extensively studied and elucidated. The unique features of the CH4 precursor (small molecular size and oxygen-free) make the resultant carbon materials well graphitized with the (002) crystal plane growing along the long axis of the carbon pore walls, which is highly desirable for enhancing electrical conductivity. Furthermore, simple thermal treatment at relatively low temperatures of 1000–1200 °C can be adopted to greatly enhance the graphitization degree of the carbon frameworks while the ordered mesostructure and high porosity are well retained, thus leading to the fabrication of highly graphitized ordered mesoporous carbon materials, even with an Ia3d symmetry. Finally, due to the integration of high surface areas, large pore volumes, regular mesopores and highly graphitic nature, these MGCs can be adopted as excellent supports for Pt nanoparticles with high dispersion and strong carbon to Pt binding, delivering a highly promising performance for electrocatalyzing oxygen reduction with greatly enhanced Pt utilization, activity, methanol tolerance and long-term stability compared to commercial activated carbon supported Pt catalysts. The ordered MGCs materials can be directly adopted as electrode materials for lithium-ion batteries, showing a high reversible capacity up to ∼350 mAh g−1 and good cyclic stability. Moreover, they can be also utilized as electrode materials without the use of any conductive additives for supercapacitors under a non-aqueous system, showing a specific capacitance of ∼40 F g−1 with excellent Coulombic efficiency and rate performance.
Acknowledgements
Financial supports from the National Science Foundation (20890123) and the State Key Basic Research Program of China, and the Discovery grants from the Australian Research Council (DP120101194, 0879769) are greatly appreciated. We appreciate Dr Wangjun Cui and Yingying Lv for experimental assistance regarding the electrochemical measurement. The authors acknowledge use of the facilities within the Monash Centre for Electron Microscopy.
Notes and references
- R. Ryoo, S. H. Joo and S. Jun, J. Phys. Chem. B, 1999, 103, 7743 CrossRef CAS.
- M. Hartmann, Chem. Mater., 2005, 17, 4577 CrossRef CAS.
- J. S. Huang, B. G. Sumpter and V. Meunier, Angew. Chem., Int. Ed., 2008, 47, 520 CrossRef CAS.
- H. S. Zhou, S. M. Zhu, M. Hibino, I. Honma and M. Ichihara, Adv. Mater., 2003, 15, 2107 CrossRef CAS.
- C. Vix-Guterl, E. Frackowiak, K. Jurewicz, M. Friebe, J. Parmentier and F. Beguin, Carbon, 2005, 43, 1293 CrossRef CAS.
- K. Ariga, A. Vinu, M. Miyahara, J. P. Hill and T. Mori, J. Am. Chem. Soc., 2007, 129, 11022 CrossRef CAS.
- Y. Fang, D. Gu, Y. Zou, Z. X. Wu, F. Y. Li, R. C. Che, Y. H. Deng, B. Tu and D. Y. Zhao, Angew. Chem., Int. Ed., 2010, 49, 7987 CrossRef CAS.
- M. E. Davis, Nature, 2002, 417, 813 CrossRef CAS.
- Z. X. Wu and D. Y. Zhao, Chem. Commun., 2011, 47, 3332 RSC.
- S. H. Joo, S. J. Choi, I. Oh, J. Kwak, Z. Liu, O. Terasaki and R. Ryoo, Nature, 2001, 412, 169 CrossRef CAS.
- A. Stein, Z. Y. Wang and M. A. Fierke, Adv. Mater., 2009, 21, 265 CrossRef CAS.
- Z. X. Wu, N. Hao, G. K. Xiao, L. Y. Liu, P. Webley and D. Y. Zhao, Phys. Chem. Chem. Phys., 2011, 13, 2495 RSC.
- W. C. Choi, S. I. Woo, M. K. Jeon, J. M. Sohn, M. R. Kim and H. J. Jeon, Adv. Mater., 2005, 17, 446 CrossRef CAS.
- Z. H. Wen, J. Liu and J. H. Li, Adv. Mater., 2008, 20, 743 CrossRef CAS.
- X. L. Ji, K. T. Lee, R. Holden, L. Zhang, J. J. Zhang, G. A. Botton, M. Couillard and L. F. Nazar, Nat. Chem., 2010, 2, 286 CrossRef CAS.
- C. D. Liang, Z. J. Li and S. Dai, Angew. Chem., Int. Ed., 2008, 47, 3696 CrossRef CAS.
- Y. Wan, H. F. Yang and D. Y. Zhao, Acc. Chem. Res., 2006, 39, 423 CrossRef CAS.
- Y. Meng, D. Gu, F. Q. Zhang, Y. F. Shi, L. Cheng, D. Feng, Z. X. Wu, Z. X. Chen, Y. Wan, A. Stein and D. Y. Zhao, Chem. Mater., 2006, 18, 4447 CrossRef CAS.
- C. D. Liang, K. L. Hong, G. A. Guiochon, J. W. Mays and S. Dai, Angew. Chem., Int. Ed., 2004, 43, 5785 CrossRef CAS.
- F. Q. Zhang, Y. Meng, D. Gu, Y. Yan, C. Z. Yu, B. Tu and D. Y. Zhao, J. Am. Chem. Soc., 2005, 127, 13508 CrossRef CAS.
- C. D. Liang and S. Dai, J. Am. Chem. Soc., 2006, 128, 5316 CrossRef CAS.
- H. I. Lee, J. H. Kim, D. J. You, J. E. Lee, J. M. Kim, W. S. Ahn, C. Pak, S. H. Joo, H. Chang and D. Seung, Adv. Mater., 2008, 20, 757 CrossRef CAS.
- J. Ding, K. Y. Chan, J. W. Ren and F. S. Xiao, Electrochim. Acta, 2005, 50, 3131 CrossRef CAS.
- S. H. Liu, R. F. Lu, S. J. Huang, A. Y. Lo, S. H. Chien and S. B. Liu, Chem. Commun., 2006, 3435 RSC.
- G. Gupta, D. A. Slanac, P. Kumar, J. D. Wiggins-Camacho, J. Kim, R. Ryoo, K. J. Stevenson and K. P. Johnston, J. Phys. Chem. C, 2010, 114, 10796 CAS.
- Z. L. Schaefer, M. L. Gross, M. A. Hickner and R. E. Schaak, Angew. Chem., Int. Ed., 2010, 49, 7045 CrossRef CAS.
- Y. Y. Shao, S. Zhang, C. M. Wang, Z. M. Nie, J. Liu, Y. Wang and Y. H. Lin, J. Power Sources, 2010, 195, 4600 CrossRef CAS.
- T. Matsumoto, T. Komatsu, H. Nakano, K. Arai, Y. Nagashima, E. Yoo, T. Yamazaki, M. Kijima, H. Shimizu, Y. Takasawa and J. Nakamura, Catal. Today, 2004, 90, 277 CrossRef CAS.
- S. Y. Wang, S. P. Jiang, T. J. White, J. Guo and X. Wang, Adv. Funct. Mater., 2010, 20, 1368 CAS.
- K. P. Gierszal, M. Jaroniec, T. W. Kim, J. Kim and R. Ryoo, New J. Chem., 2008, 32, 981 RSC.
- C. M. Yang, C. Weidenthaler, B. Spliethoff, M. Mayanna and F. Schüth, Chem. Mater., 2005, 17, 355 CrossRef CAS.
- C. H. Huang, R. A. Doong, D. Gu and D. Y. Zhao, Carbon, 2011, 49, 3055 CrossRef CAS.
- A. H. Lu, W. C. Li, E. L. Salabas, B. Spliethoff and F. Schüth, Chem. Mater., 2006, 18, 2086 CrossRef CAS.
- H. F. Yang, Y. Yan, Y. Liu, F. Q. Zhang, R. Y. Zhang, Y. Meng, M. Li, S. H. Xie, B. Tu and D. Y. Zhao, J. Phys. Chem. B, 2004, 108, 17320 CrossRef CAS.
- K. T. Lee, X. L. Ji, M. Rault and L. F. Nazar, Angew. Chem., Int. Ed., 2009, 48, 5661 CrossRef CAS.
- T. W. Kim, I. S. Park and R. Ryoo, Angew. Chem., Int. Ed., 2003, 42, 4375 CrossRef CAS.
- S. B. Yoon, G. S. Chai, S. K. Kang, J.-S. Yu, K. P. Gierszal and M. Jaroniec, J. Am. Chem. Soc., 2005, 127, 4188 CrossRef CAS.
- Y. Meng, D. Gu, F. Q. Zhang, Y. F. Shi, H. F. Yang, Z. Li, C. Z. Yu, B. Tu and D. Y. Zhao, Angew. Chem., Int. Ed., 2005, 44, 7053 CrossRef CAS.
- Y. D. Xia and R. Mokaya, Adv. Mater., 2004, 16, 1553 CrossRef CAS.
- C. J. Chen and M. H. Back, Carbon, 1979, 17, 175 CrossRef CAS.
- P. Lucas and A. Marchand, Carbon, 1990, 28, 207 CrossRef CAS.
- Z. X. Wu, Y. X. Yang, D. Gu, Y. P. Zhai, D. Feng, Q. Li, B. Tu, P. A. Webley and D. Y. Zhao, Top. Catal., 2009, 52, 12 CrossRef CAS.
- X. S. Li, C. W. Magnuson, A. Venugopal, R. M. Tromp, J. B. Hannon, E. M. Vogel, L. Colombo and R. S. Ruoff, J. Am. Chem. Soc., 2011, 133, 2816 CrossRef CAS.
- Q. K. Yu, L. A. Jauregui, W. Wu, R. Colby, J. F. Tian, Z. H. Su, H. L. Cao, Z. H. Liu, D. Pandey, D. G. Wei, T. F. Chung, P. Peng, N. P. Guisinger, E. A. Stach, J. M. Bao, S. S. Pei and Y. P. Chen, Nat. Mater., 2011, 10, 443 CrossRef CAS.
- D. Y. Zhao, Q. S. Huo, J. L. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS.
- F. Kleitz, S. H. Choi and R. Ryoo, Chem. Commun., 2003, 2136 RSC.
- Z. X. Wu, Y. X. Yang, D. Gu, Q. Li, D. Feng, Z. X. Chen, B. Tu, P. A. Webley and D. Y. Zhao, Small, 2009, 5, 2738 CrossRef CAS.
- Such conditions refer to the following heating procedure: heating the sample from room temperature directly to 900–1200 °C with a heating rate of 20 °C min−1 and keeping isothermal at the final temperature for 2 h.
- Z. X. Wu, Y. Meng and D. Y. Zhao, Microporous Mesoporous Mater., 2010, 128, 165 CrossRef CAS.
- S. N. Che, K. Lund, T. Tatsumi, S. Iijima, S. H. Joo, R. Ryoo and O. Terasaki, Angew. Chem., Int. Ed., 2003, 42, 2182 CrossRef CAS.
- S. Jun, S. H. Joo, R. Ryoo, M. Kruk, M. Jaroniec, Z. Liu, T. Ohsuna and O. Terasaki, J. Am. Chem. Soc., 2000, 122, 10712 CrossRef CAS.
- C. Cioffi, S. Campidelli, C. Sooambar, M. Marcaccio, G. Marcolongo, M. Meneghetti, D. Paolucci, F. Paolucci, C. Ehli, G. M. A. Rahman, V. Sgobba, D. M. Guldi and M. Prato, J. Am. Chem. Soc., 2007, 129, 3938 CrossRef CAS.
- C. W. Huang, C. H. Hsu, P. L. Kuo, C. T. Hsieh and H. S. Teng, Carbon, 2011, 49, 895 CrossRef CAS.
- X. Yu and S. Ye, J. Power Sources, 2007, 172, 133 CrossRef CAS.
- A. Ignaszak, S. Y. Ye and E. Gyenge, J. Phys. Chem. C, 2009, 113, 298 CAS.
- H. Y. Wang, T. Abe, S. Maruyama, Y. Iriyama, Z. Ogumi and K. Yoshikawa, Adv. Mater., 2005, 17, 2857 CrossRef CAS.
- W. C. Li, G. Z. Nong, A. H. Lu and H. Q. Hu, J. Porous Mater., 2011, 18, 23 CrossRef CAS.
- J. Chmiola, G. Yushin, Y. Gogotsi, C. Portet, P. Simon and P. L. Taberna, Science, 2006, 313, 1760 CrossRef CAS.
- H. J. Liu, X. M. Wang, W. J. Cui, Y. Q. Dou, D. Y. Zhao and Y. Y. Xia, J. Mater. Chem., 2010, 20, 4223 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: details regarding the Koutecky-Levich equation; comprehensive data of TG curves, N2 sorption, SEM and TEM images of the MGCs obtained by the graphitization promotion at 1000–1200 °C; SAXS, N2 sorption results, and TEM images of the MGCs templated from the mesoporous silica KIT-6; N2 sorption results, SEM and TEM images of the MGC-supported and the commercial activated-carbon-supported Pt electrocatalysts; CV curves and polarization profiles before and after 100 cycles of the MGC-supported and the commercial activated-carbon-supported Pt electrocatalysts recorded under N2 and O2-saturated H2SO4 electrolyte in the presence of 0.5 M CH3OH. See DOI: 10.1039/c2jm30192j |
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume). Then an aqueous solution of Pt(NH3)4Cl2·xH2O with a calculated Pt loading of ∼4 wt% was added, and it was sonicated for 30 min. Then, the solvent was evaporated at 50 °C under continuous stirring. After drying, the composite was heated to 900 °C with a heating rate of 2 °C min−1 and held for 3 h under an Ar atmosphere to carbothermally reduce the Pt precursor to metallic Pt nanoparticles. After cooling, the final electrocatalyst for oxygen reduction was obtained. The final Pt content was determined to be ∼4.7 wt%. A commercial activated carbon supported Pt electrocatalyst with ∼5.6 wt% of Pt, highly dispersive in nature, was used as a counterpart for comparison. Its structure and porosity, as well as the size and distribution of the Pt nanoparticles, were well characterized and can be found in the ESI.†
1 by volume). Then an aqueous solution of Pt(NH3)4Cl2·xH2O with a calculated Pt loading of ∼4 wt% was added, and it was sonicated for 30 min. Then, the solvent was evaporated at 50 °C under continuous stirring. After drying, the composite was heated to 900 °C with a heating rate of 2 °C min−1 and held for 3 h under an Ar atmosphere to carbothermally reduce the Pt precursor to metallic Pt nanoparticles. After cooling, the final electrocatalyst for oxygen reduction was obtained. The final Pt content was determined to be ∼4.7 wt%. A commercial activated carbon supported Pt electrocatalyst with ∼5.6 wt% of Pt, highly dispersive in nature, was used as a counterpart for comparison. Its structure and porosity, as well as the size and distribution of the Pt nanoparticles, were well characterized and can be found in the ESI.†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume). The cell was assembled from the as-prepared working electrode, lithium negative electrode, and separators made of a Celgard 2300 film. Charge/discharge experiments were conducted at a rate of 0.1 C between 0.01 and 3.0 V using a LAND CT2001A Battery Cycler (Wuhan, China). Lithium insertion into the working electrode was referred to as discharge and extraction as charge. CV measurements were carried out on a CHI Instruments electrochemical workstation (model 600A) with a scan rate of 1 mV s−1. The metallic lithium was used as counter and reference electrode.
1 by volume). The cell was assembled from the as-prepared working electrode, lithium negative electrode, and separators made of a Celgard 2300 film. Charge/discharge experiments were conducted at a rate of 0.1 C between 0.01 and 3.0 V using a LAND CT2001A Battery Cycler (Wuhan, China). Lithium insertion into the working electrode was referred to as discharge and extraction as charge. CV measurements were carried out on a CHI Instruments electrochemical workstation (model 600A) with a scan rate of 1 mV s−1. The metallic lithium was used as counter and reference electrode.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in weight ratio) were well mixed without the use of any conductive additive such as acetylene black in order to make the high conductivity of the MGCs stand out. The slurries were coated on an aluminum foil which was employed as current collectors for both positive and negative electrodes. After coating, the electrode was dried at 80 °C for 10 min to remove the solvent before pressing. The electrode was cut into disks of ∼1.2 cm in diameter, vacuum-dried at 100 °C for 24 h, and weighed. The typical active mass loading on the electrode was ∼5 mg cm−2. The electrolyte was 1.0 M (C2H5)4NBF4 in propylene carbonate (Honeywell Corp). CV curves and galvanostatic charge/discharge profiles were measured on a CHI 760 electrochemical working station under ambient conditions.
10 in weight ratio) were well mixed without the use of any conductive additive such as acetylene black in order to make the high conductivity of the MGCs stand out. The slurries were coated on an aluminum foil which was employed as current collectors for both positive and negative electrodes. After coating, the electrode was dried at 80 °C for 10 min to remove the solvent before pressing. The electrode was cut into disks of ∼1.2 cm in diameter, vacuum-dried at 100 °C for 24 h, and weighed. The typical active mass loading on the electrode was ∼5 mg cm−2. The electrolyte was 1.0 M (C2H5)4NBF4 in propylene carbonate (Honeywell Corp). CV curves and galvanostatic charge/discharge profiles were measured on a CHI 760 electrochemical working station under ambient conditions.













