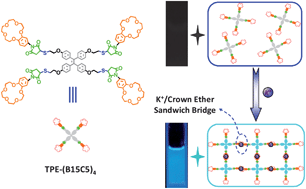
In this work, we integrated the concept of aggregation-induced emission (AIE) with the specific supramolecular recognition between K+ ions and crown ether moieties to develop more effective fluorometric K+ probes. We synthesized a novel crown ether-functionalized tetraphenylethene (TPE) derivative, TPE-(B15C5)4, via the thiol-ene click reaction of thiol-derivatized TPE, TPE-(SH)4, with maleimide-functionalized benzo-15-crown-5 (B15C5). In TPE-(B15C5)4, the TPE core and four outer B15C5 moieties serve as the AIE-active motif and supramolecular K+-recognizing functionalities, respectively. TPE- (B15C5)4 molecularly dissolves in THF with negligible fluorescence emission. As we have envisaged, upon K+ addition, TPE-(B15C5)4 can be effectively induced to aggregate due to K+-mediated cross-linking via the formation of K+/B15C5 (1/2 molar ratio) molecular recognition complex in a sandwiched manner. This process is concomitantly accompanied with the turn-on of fluorescence emission via the AIE mechanism. Thus, TPE-(B15C5)4 can serve as highly sensitive and selective fluorometric off–on K+ probes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...