Effect of the addition of non-ionic surfactants on the emission characteristic of direct current atmospheric pressure glow discharge generated in contact with a flowing liquid cathode
Krzysztof
Greda
,
Piotr
Jamroz
and
Pawel
Pohl
*
Wroclaw University of Technology, Faculty of Chemistry, Division of Analytical Chemistry, Wybrzeze Stanislawa Wyspianskiego 27, 50-370 Wroclaw, Poland. E-mail: pawel.pohl@pwr.wroc.pl; Fax: +48-71-320-2494; Tel: +48-71-320-3445
First published on 12th November 2012
Abstract
A direct current atmospheric pressure glow discharge generated in contact with a flowing liquid cathode was used to study the effect of the concentration of two non-ionic surfactants, i.e., Triton x-45 and Triton x-405 added to electrolyte solutions, on the emission characteristic of the excitation source by using optical emission spectrometry. The emission intensity of different molecular and atomic species as well as the background level were measured. Selected spectroscopic parameters, i.e., rotational temperatures of OH and N2 molecules, excitation temperatures of Co and H atoms, the electron number density and the intensity ratio for Mg II to Mg I lines, were also determined. The net intensity of atomic emission lines of several metals (Cs, Cu, Hg, Mg, Mn and Pb) was found to be enhanced by more than 4 times in the presence of the heavier surfactant in solution at the concentration corresponding to 5 times its critical micelle concentration. Coincidently, the intensity of the background in the vicinity of these lines and its fluctuation were also significantly decreased. Possible changes in sputtering, collisional-recombination and excitation processes that may occur in the near-cathode zone of the discharge are discussed and the phenomenon explained.
1 Introduction
Direct current (dc) atmospheric pressure glow discharge (APGD) generated in contact with a flowing liquid cathode is now considered to be one of the most promising alternative miniaturized excitation sources that can be applied in process control or environmental monitoring studies for the direct trace element analysis of various liquid samples.1–6 This is primarily due to the expedient emission characteristic of this new excitation source, i.e., relatively simple atomic emission spectra containing the most prominent atomic lines and less common spectral overlaps of these lines. Such a distinctive feature of dc-APGD generated in contact with the liquid cathode makes it highly desirable for direct and on-line optical emission spectrometric (OES) determinations of trace metal impurities in different sample solutions.7–11 In addition, the inherent nature of this miniaturized discharge brings power density between electrolyte solutions serving as the cathode and the counter electrode that is about one order of magnitude or more higher than the density power established for commercially available inductively coupled plasma (ICP) sources.3 As a result, overwhelming in analytical applications of this excitation source, quantitative measurements by OES are possible with reasonably good detectability, commonly sub-mg l−1 range, is attained. Decent precision and the reproducibility of signals is better than several %, while the consumption of discharge gases is incomparably smaller; even no gas supply is needed since the discharge is fully operable in air.12,13 This in turn means little effort and low operating costs are required to sustain and control the discharge.1,2,4,9,14 Electrical power consumed by dc-APGD generated in contact with the liquid cathode typically ranges from several to tens Watts and is mostly dissipated in the near-cathode region of the discharge to evaporate the electrolyte solution and sustain important processes and reactions occurring at the gas–liquid interface.1–6The progress made in the last decade in terms of the design of discharge cells for this type of excitation source has led to an increase in the concentration of atoms in the near-cathode region of the discharge and has strict implications for the improvement of analytical performance of APGD generated in contact with the liquid cathode.1–6,9,15–21 Correspondingly, the latest modifications, consisting changes in the construction and the geometry of capillaries used for the introduction of electrolyte solutions, are reflected by enhanced optical thinness of the discharge, its better stability and much greater reproducibility of the discharge gap.1–4,18–20 As a result, improved detection limits of metals, commonly in the range of 0.001–0.01 mg l−1, linearity concentration ranges from 2 to at least 4 orders of the magnitude and higher precision, in most cases better than 6%, are achievable, showing the real attractiveness and the potential of APGD generated in contact with the liquid cathode for trace element analysis by OES.
It seems, however, that improvement of the emission characteristic and therefore analytical performance of these APGD systems can also be achieved through modification to the composition and physicochemical properties of electrolyte solutions serving as liquid cathodes. It has been recently reported that the addition of low molecular weight organic compounds, i.e., formic acid or acetic acid at concentrations not higher than 10% (v/v), results in a 3- to 4-fold increase in emission signals for Hg as compared to those obtained in conditions without the addition of both organic compounds to electrolyte solutions.20 The signal enhancement observed is thought to be due to changes in the boiling point and surface tension of electrolyte solutions, however, it is probable that the H and CO radicals originating from the decay of these low molecular organic acids are also involved in the reduction of Hg(II) ions to Hg(0) vapors.22
Considering the self-organizing properties of surfactant assemblies reported in analytical atomic spectrometry,23 including the possibility to change the rate of chemical reactions and the distribution of resulting reaction products, it can be expected that the presence of these compounds will enhance sputtering efficiency and the transfer rate of metals to the near-cathode zone of the discharge. To the best of our knowledge emission characteristic and performance of APGD generated in contact with the liquid cathode and modified by the addition of surfactants to electrolyte solutions have not yet been studied. The investigation of the behavior of such a discharge system is important and is the objective of the present contribution. Herein, two non-ionic, i.e. a light, Triton x-45, and a heavy, Triton x-405, surfactants were selected and the effect of their addition to electrolyte solutions serving as liquid cathodes in dc-APGD on the emission characteristic of the discharge was studied by considering first changes in the morphology of the band and the atomic emission spectra. Then, the influence of the concentration of these surfactants on selected spectroscopic parameters, i.e., rotational temperatures determined from rotational-vibrational structures of OH and N2 bands (Trot(OH), Trot(N2)), excitation temperatures measured with H and Co I lines (Texc(H), Texc(Co I)), the electron number density evaluated on the basis of the Stark broadening effect of the Hβ 486.1 nm line, and the ratio of intensities of ionic to atomic lines of Mg (IMg II/IMg I), was examined to predict changes in sputtering, collisional-recombination and excitation processes that may occur in the near-cathode zone of the discharge and which are closely related to the observed improvement in the performance of dc-APGD in reference to the atomic emission characteristic of the studied metals.
2 Experimental
2.1 Excitation source and radiation acquisition
The dc-APGD excitation source with a flowing liquid cathode used in this work is described elsewhere.3 In brief, a stable discharge was sustained in the open-to-air atmosphere after applying a high dc voltage (1500 V) to vertically arranged in a line electrodes, i.e., a molybdenum rod (OD 2 mm), acting as the anode, and an electrolyte solution overflowing a graphite tube (OD 6 mm, ID 4 mm), acting as the cathode. Electrolyte solutions were pumped at a flow rate of 1.2 ml min−1 using a two channel peristaltic pump (LabCraft, France) and were introduced to the cathode compartment of a glass housing through a quartz tube (OD 4 mm, ID 2 mm). This tube was inserted into the graphite tube in such a way that its edge was 4 mm below the edge of the graphite tube. Overflowing electrolyte solutions were collected in a reservoir formed at the bottom of the housing and instantly drained using a peristaltic pump. A positive potential was applied to the molybdenum rod. The electric contact was provided through a platinum wire, directly bonded to the graphite tube. The discharge gap of 5 mm was used but it was possible to regulate it by a micrometer screw to which the molybdenum rod was attached. A 10 kΩ ballast resistor was immersed in the electric circuit of the discharge to stabilize the discharge current (30 mA).All emission spectra of the discharge in the range from 190 to 900 nm were recorded using a 320 mm focal length single grating imagining spectrometer Triax 320 (HORIBA Jobin Yvon), equipped with a 1200 grooves mm−1 holographic grating and a Hamamatsu R-928 photomultiplier. The radiation of the near-cathode zone of the discharge was extracted through a built-in horizontal diaphragm obscuring the entrance slit (height 1.0 mm, width 100 μm) of the spectrometer and focused by using an achromatic UV lens. Emission spectra were recorded with an integration time of 0.1 s and a 0.02 nm interval. The exit slit width of the spectrometer was 100 μm, while a voltage of −700 V was used to bias the photomultiplier. The output signal of the photomultiplier was amplified using a HORIBA Jobin Yvon SpectraAcq2 single photon counting acquisition system. A SpectraMax/32 for Windows software (Instruments SA, Inc.), version 3.2, was used to handle the spectrometer and control its configuration as well as acquire and process the data.
2.2 Reagents and solutions
Doubly distilled water was used throughout. Single-element 1000 mg l−1 standard solutions of Ca, Cd, Co, Cr, Cs, Cu, Fe, Hg, Li, Mg, Mn, Ni, Pb, Rb, Sr and Zn were supplied by Sigma-Aldrich Chemie GmbH (Germany). Non-ionic surfactants, including Triton x-45 [4-(1,1,3,3-tetramethylbutyl)phenyl-polyethylene glycol, C14H22O(C2H4O)5] and a 70% water solution of Triton x-405 [polyethylene glycol tert-octylphenyl ether, C14H22O(C2H4O)40] were also provided by Sigma-Aldrich. Single-element or mixed working standard solutions of studied metals at concentrations within the range from 1.0 to 25 mg l−1 were prepared by appropriate dilutions of bulk standard solutions. All these solutions were acidified using a concentrated HCl solution (ACS reagent, 36.5–38.0%) obtained from J. T. Baker. The final concentration of HCl in these solutions was 0.1 mol l−1. Surfactants were separately added to working solutions and their final concentrations corresponded to ¼, ½, ¾, 1, 1½, 2, 3, 4 and 5 times their critical micelle concentrations (CMCs), i.e., 0.10 and 0.81 mmol l−1, respectively for Triton x-45 and Triton x-405.242.3 Measurements of spectroscopic parameters
Rotational temperatures were assessed using unresolved rotational-vibrational spectra of OH and N2 molecules and the method of the fitting of these experimental spectra with spectra simulated at different rotational temperatures by means of Lifbase25 (for band emission spectra of the OH molecule) and Specair26 (for band emission spectra of the N2 molecule) computer programs, is described elsewhere.21,27 For the calculation of Trot(OH), the (0-0) band of the OH A2Σ+→X2Π system with the band head at 309.3 nm was selected and its emission spectra was acquired in the range from 300 to 325 nm. Trot(N2), the (0-2) band of the N2 C3Πu→B3Πg system with the band heads at 380.5 nm the emission spectra were recorded in the range from 376 to 381 nm. Excitation temperatures were determined using a two-line intensity ratio method. Texc(H), Hα 656.3 nm and Hβ 486.1 nm lines with excitation energies of 12.1 and 12.7 eV, respectively, were selected as described elsewhere.21,27Texc(Co I), Co I 358.7 nm (the excitation energy of 4.50 eV) and Co I 359.5 nm (3.62 eV) lines were taken. Spectroscopic data for these lines, i.e., energies of upper-state levels, transition probabilities and statistical weights of upper- and lower-state levels, used in calculations are published in NIST Atomic Spectra Database.28 All temperatures were averaged on the basis of three repeated measurements of emission spectra under given experimental conditions. The precision of the estimation of Trot(OH) and Trot(N2) was within 1.0–6.1% while in case of the determination of Texc(H) and Texc(Co I) it was within 0.8–6.5%.The Stark broadening (λS, in nm) of the Hβ 486.1 nm line was used for the determination of ne (in cm−3). To take its values, experimental profiles of the Hβ line, averaged for seven consecutive measurements, were fitted at first with a Voight function using a Galactic Grams/32 computer program. A Lorentzian part of full widths at half maximum (FWHMs) of resulting line profiles was corrected for the instrumental broadening and used in the formula given by Gigososa et al.:29 (λS/4.800) = (ne/1017)0.68116. The precision of the estimation of ne, based on the standard deviation of seven repeated measurements of the line profile, was better than 10%. Finally, to follow changes in excitation conditions in the discharge, ionic to atomic intensity ratios for Mg emission lines, i.e., Mg II 279.6 nm (the excitation energy of 4.43 eV) to Mg I 285.2 nm (4.42 eV), were determined. Intensities of these lines were measured three times and averaged for calculations of IMg II/IMg I ratios. The precision of the determination of this parameter was better than 7.0%.
3 Results and discussion
3.1 Effect of the addition of surfactants on band emission spectra
The morphology of emission spectra of the dc-APGD source generated in contact with a multi-element standard solution containing Triton x-45 or Triton x-405 and a solution without the surfactant was found to considerably differ over the whole spectral range. It was particularly noticeable within the range of 190–700 nm and in case of the addition of Triton x-405 at the concentration corresponding to its 5 × CMC (see Fig. 1). Apparently, in the presence of this surfactant in the electrolyte solution intensive (2-0), (1-0), (0-0), (0-1), (0-2) and (0-3) bands of the third positive A2Σ+→X2Π system of the NO molecule, identified in the emission spectrum of dc-APGD generated in contact with the solution without it (band heads at 205.3, 215.5, 226.9, 237.0, 247.9 and 259.6 nm, respectively), completely disappeared or were significantly reduced. What is more, the magnitude of these changes was established to be strictly dependent on the concentration of the surfactant in the electrolyte solution delivered to the discharge system and whether the electrolyte solution contained heavier Triton x-405 or its lighter analogue Triton x-45. In general, stronger decreases in the intensity of the band heads were found to occur for Triton x-405. When compared to conditions without the addition of surfactants into electrolyte solutions, the net intensity of the (0-1) band head of the NO A2Σ+→X2Π system at 247.9 nm and the background level in the vicinity of this band rapidly declined (by about 90% and 40%, respectively) when Triton x-405 was already present in the electrolyte solution at the concentration corresponding to its ¼ × CMC. An increase in the concentration of this surfactant to its 5 × CMC was established to neither result in a further reduction of the net intensity of this band head nor the background level. When lighter Triton x-45 was used, a drop in the net intensity of the (0-1) NO band head and the background level in the vicinity of this band was also substantial but not as fast and high. Here, a gradual decrease in the net intensity of the band head was found to reach its maximal value (a reduction by about 80%) at the concentration of the surfactant in the electrolyte solution corresponding to its 2 × CMC. An increase in the concentration of this surfactant to its 5 × CMC did not result in further changes of the band head net intensity. A similar gradual decrease was observed for the background level of this band, but the highest 30% fall of the background intensity was noted at the concentration of Triton x-45 corresponding to its 2 × CMC and essentially remained at the same level when the concentration of this surfactant was increased to its 5 × CMC. | ||
| Fig. 1 Emission spectra of dc-APGD generated in contact with electrolyte solutions containing Ca, Cd, Cs, Cu, Hg, Li, Mg, Pb, Rb, Sr and Zn without (black line) and with (gray line) the addition of Triton x-405 at the concentration corresponding to its 5 × CMC. | ||
Gradual but less intensive, when compared to NO bands, drops in intensities of other bands identified in emission spectra of the discharge fed with electrolyte solutions containing Triton x-45 or Triton x-405 were observed, i.e., for (2-0), (1-0) and (0-0) bands of the OH A2Σ+→X2Π system at spectral ranges of 260–270 nm, 280–290 nm and 306–320 nm, respectively, and (4-2), (3-1), (2-0), (0-0), (2-3), (1-2), (0-1), (3-5), (2-4), (1-3), (0-2), (3-6), (2-5) and (0-3) bands of the N2 C3Πu→B3Πg system with band heads at 295.3, 296.2, 297.7, 337.1, 350.0, 353.7, 357.7, 367.2, 371.0, 375.5, 380.5, 389.5, 394.3 and 405.9 nm, respectively. Again, the magnitude of the intensity reduction was found to be associated with the concentration of the surfactant used and its size. Accordingly, the net intensity of the (0-0) band head of the OH A2Σ+→X2Π system at 309.3 nm was established to be more strongly diminished when Triton x-405 was present in electrolyte solutions. In this case, a 60 ± 1% reduction (the average value for its 1 × CMC, 1½ × CMC and 2 × CMC) of the net intensity of this band head was determined. When Triton x-45 was added to electrolyte solutions at the concentration corresponding to its 1 × CMC, 1½ × CMC and 2 × CMC, only a 25±2% decrease was noted. A further increase in the amount of both surfactants (up to the concentration corresponding to their 5 × CMCs) did not cause any significant changes in the net intensity of the OH band head. A similar trend was also observed for the (0-1) band head of the N2 C3Πu→B3Πg system, i.e., higher drops of the net intensity of the N2 band head and the background level in the vicinity of this band were distinguished when the liquid cathode contained Triton x-405 instead of Triton x-45. When compared to conditions without the addition of the surfactant to electrolyte solution, an average reduction of the net intensity of the N2 band head, considering values obtained when the surfactant was added to electrolyte solutions at concentrations corresponding to its 1 × CMC, 1½ × CMC, 2 × CMC, 3 × CMC, 4 × CMC and 5 × CMC, was by 55±5% and 30±5%, respectively, for Triton x-405 and Triton x-45. At the same time, a corresponding average reduction of the background level in the vicinity of this band was 25±2% (Triton x-405) and 10±1% (Triton x-45).
In conditions with the addition of surfactants to electrolyte solutions the only molecules produced in the discharge and found to increase the intensity of their bands in the emission spectrum were those occurring in the range of 548–588 nm, 594–614 nm, 614–634 nm and 652–680 nm. Using single-element standard solutions it was possible to identify these molecules as CaOH, SrOH, CaO and SrCl.30 The decomposition of both non-ionic surfactants was very likely to be due to the influence of ionizing reduction conditions, i.e., the presence of hydrated electrons, H atoms, OH radicals and H2O2 molecules.31 Nevertheless, the emission of any molecular and atomic carbon species, i.e., bands of CN, CH, C2, CO or CO+ and atomic lines of C, was not found in emission spectra of dc-APGD fed with electrolyte solutions containing Triton x-45 or Triton x-405. Such carbon radicals were either not identified in emission spectra of dc-APGD in case of the addition of formic or acetic acids (at the 1–20% v/v level) to electrolyte solutions.20
Additionally, intensities of other fixed atomic components of the emission spectrum of dc-APGD generated in contact with the liquid cathode, i.e., Hα and Hβ lines, O I lines at 777.2, 777.4 and 844.6 nm in addition to the O II 441.5 nm line, were found to drop when studied surfactants were added to electrolyte solutions. This can be seen in Fig. 2, where the behavior of the relative net intensity (in reference to the highest value in a series) of Hα 656.2 nm (the excitation energy of 12.1 eV), O I 777.2 nm (10.7 eV) and O II 441.5 nm (26.2 eV) lines versus the concentration of the surfactant in the electrolyte solution is given. Similarly as in case of band spectra of NO, OH and N2 radicals, a difference in the magnitude of the reduction of net intensities of Hα, O I and O II lines due to the concentration of the surfactant in the solution and its size was observed. A higher deterioration of net intensities of H and O lines were established in case of the addition of Triton x-405, particularly in case of the O I line. The reduction of the background intensity established for these lines was also higher in case of Triton x-405. For example, for O I and O II lines, the background level in the vicinity of these lines was found to gradually reduce (maximally by 5% and 20%, respectively) when the concentration of Triton x-405 increased to its 2 × CMC and then, with a further increase in the concentration of the surfactant, practically remained at the same level. When Triton x-45 was added to electrolytes solutions, a steady decrease in the background intensity of O I and O II lines was noted as well and attained 5% and 10%, correspondingly, at the concentration of the surfactant corresponding to its 1 × CMC.
 | ||
| Fig. 2 Effect adding Triton x-45 (dotted line) and Triton x-405 (solid line) on the relative net intensity of Hα 656.2 nm, O I 777.2 nm and O II 441.5 nm lines. Results are average values for 3 measurements. | ||
3.2 Effect of the addition of surfactants on atomic emission spectra of metals
The sputtering of the surface of the liquid cathode is presumed to be responsible for a strong atomic emission of metals (Ca, Cd, Co, Cr, Cs, Cu, Fe, Hg, Li, Mg, Mn, Ni, Pb, Rb, Sr and Zn) present in multi-element working standard solutions. The most prominent atomic lines of these metals, i.e., Ca I 422.7 nm, Cd I 228.8 nm, Co I 240.7 nm, Cr I 359.3 nm, Cs I 852.1 nm, Cu I 324.8 nm, Fe I 248.3 nm, Hg I 253.7 nm, Li I 670.8 nm, Mg I 285.2 nm, Mn I 279.5 nm, Ni I 232.0 nm, Pb I 368.3 nm, Rb I 780.0 nm, Sr I 460.7 and Zn I 213.9 nm, were identified in emission spectra of dc-APGD fed with electrolyte solutions containing ions of these metals and admixed Triton x-45 or Triton x-405. In addition, due to the lower background level and the intensity of molecular bands, the presence of surfactants made the less intensive atomic lines of metals such as Cr (at 357.9, 360.5, 425.4, 427.5, and 429.0 nm), Mn (at 257.6, 259.4, 260.6, 279.8, 280.1, 382.4 and 383.3 nm), Fe (at 238.2, 239.6, 246.3, 247.3, 248.8, 249.1, 251.1, 252.3, 252.7, 259.9, 272.1, 302.1, 344.1, 360.1, 361.9, 363.1, 364.8, 372.0, 373.5, 374.6, 382.0, 382.6 and 386.0 nm), Co (at 241.5, 242.5, 243.2, 243.7, 243.9, 251.1, 252.1, 252.9, 254.4, 304.4, 339.6, 340.5, 340.9, 341.3, 341.7, 343.2, 344.4, 344.9, 345.4, 346.3, 346.6, 347.4, 348.9, 349.6, 350.2, 350.6, 351.0, 351.3, 351.8, 352.3, 352.7, 353.0, 353.3, 356.9, 357.5, 358.7, 359.5, 360.2, 360.5, 384.5, 387.4, 389.4, 393.6 and 399.5 nm) or Ni (at 229.0, 230.1, 231.2, 231.7, 232.1, 232.6, 233.8, 234.6, 299.4, 300.2, 300.4, 301.2, 303.8, 305.1, 305.4, 305.8, 337.0, 338.1, 339.3, 341.5, 342.4, 343.4, 343.7, 344.6, 345.3, 345.8, 346.2, 347.3, 349.3, 351.0, 351.5, 352.5, 356.6, 359.7, 361.0, 361.9 and 385.8 nm) visible. Ionic lines of Mg (at 279.6 and 280.3 nm), Ca (at 393.4 and 396.8 nm) and Sr (at 407.8 and 421.6 nm) were also identified.The most significant observation, however, was that the addition of surfactants to electrolyte solutions improved the analytical performance of the discharge system. Firstly, the addition of Triton x-45 and particularly of Triton x-405 (see Fig. 1) was found to decrease the background intensity of atomic emission lines of studied metals and substantially reduce the background fluctuation (expressed as the relative standard deviation of the background intensity in the vicinity of the line). As can be seen from Table 1, the addition of Triton x-405 was especially beneficial for atomic emission lines occurring in the spectral range of 200–500 nm and which had excitation energies higher than 3 eV but lower than 5 eV (Ca, Cd, Co, Cr, Cu, Fe, Hg, Mg, Mn, Ni, Pb and Zn). When compared to conditions without the presence of the surfactant in solution, the background level following the addition of Triton x-405 to the electrolyte solution at a concentration corresponding to its 1 × CMC and 5 × CMC was noted to decrease from about 20% (Cr, Sr) to over 50% (Mg, Zn). At the same time the fluctuation of the background intensity considerably lowered. Secondly, a relatively high amplification of net intensities of atomic emission lines of studied metals was observed, especially for Cs, Cu, Hg, Mg, Mn and Pb, of more than 4-fold (see results in Table 2). When compared to net intensities of atomic emission lines of studied metals recorded in conditions without the presence of a surfactant in the electrolyte solution, the magnitude of the intensity amplification was established to depend on the concentration of the surfactant and its size. The highest increases were found for Triton x-405 present in the electrolyte solution at the concentration corresponding to its 5 × CMC but, in most cases, amplification factors established under these conditions were comparable to those achieved when the concentration of the surfactant corresponded to its 3 × CMC and 4 × CMC, respectively.
| Analytical line, nm (Excitation energy, eV) | Background intensity decreasea, % and (background fluctuation decreaseb, %) | |
|---|---|---|
| 1 × CMC | 5 × CMC | |
| a Average values for 10 measurements of the background. b Relative standard deviation of the background within ±0.6 nm from the center of the line profile. | ||
| Ca I 422.7 (2.93) | 26 (59) | 24 (60) |
| Cd I 228.8 (5.41) | 45 (77) | 39 (70) |
| Co I 240.7 (5.15) | 41 (73) | 41 (61) |
| Cr I 359.3 (3.45) | 19 (40) | 17 (31) |
| Cs I 852.1 (1.45) | 1.7 (4.3) | 1.2 (11) |
| Cu I 324.8 (3.82) | 31 (70) | 31 (60) |
| Fe I 248.3 (4.99) | 33 (72) | 20 (69) |
| Hg I 253.7 (4.88) | 40 (80) | 26 (76) |
| Li I 670.8 (1.85) | 3.9 (43) | 1.1 (49) |
| Mg I 285.2 (4.34) | 56 (54) | 55 (66) |
| Mn I 279.5 (4.44) | 43 (82) | 36 (79) |
| Ni I 232.0 (5.34) | 39 (85) | 26 (82) |
| Pb I 368.3 (4.34) | 28 (48) | 36 (23) |
| Rb I 780.0 (1.59) | 4.9 (20) | 4.6 (11) |
| Sr I 460.7 (2.69) | 21 (49) | 24 (53) |
| Zn I 213.9 (5.80) | 61 (90) | 39 (61) |
| Analytical line, nm | Triton x-45 | Triton x-405 | ||
|---|---|---|---|---|
| 1 × CMC | 5 × CMC | 1 × CMC | 5 × CMC | |
| a Net intensities of atomic emission lines were measured 10 times and averaged. | ||||
| Ca 422.7 | 0.8 | 0.9 | 0.9 | 2.6 |
| Cd 228.8 | 1.6 | 1.8 | 2.6 | 3.3 |
| Co 240.7 | 1.7 | 2.0 | 2.3 | 3.4 |
| Cr 359.3 | 1.7 | 1.9 | 2.4 | 3.7 |
| Cs 852.1 | 1.0 | 1.1 | 1.9 | 4.2 |
| Cu 324.8 | 1.6 | 1.6 | 3.8 | 6.2 |
| Fe 248.3 | 1.6 | 2.1 | 1.6 | 3.7 |
| Hg 253.7 | 1.5 | 1.6 | 2.3 | 4.7 |
| Li 670.8 | 0.7 | 0.7 | 0.8 | 2.0 |
| Mg 285.2 | 1.6 | 1.8 | 2.0 | 4.2 |
| Mn 279.5 | 1.2 | 1.4 | 2.0 | 4.9 |
| Ni 232.0 | 1.3 | 1.9 | 1.7 | 3.2 |
| Pb 368.3 | 1.4 | 2.1 | 6.0 | 8.8 |
| Rb 780.0 | 1.3 | 1.4 | 1.6 | 3.5 |
| Sr 460.7 | 0.6 | 0.8 | 1.1 | 2.4 |
| Zn 213.9 | 1.2 | 1.2 | 1.2 | 2.2 |
Considering the above information on the emission characteristic of band and atomic emission spectra assessed for dc-APGD generated in contact with electrolyte solutions containing Triton x-45 or Triton x-405, it seems reasonable that the addition of these surfactants may have a dual action. On one hand, by reducing the dynamic surface tension of electrolyte solutions, conditions are favorable for the detachment of small drops from the surface and the sputtering of solution components may result. This is the case where Triton x-405 is added to water at its 1 × CMC which results in the reduction of the dynamic surface tension of the solution by 25% when compared to the value of water (72 mN m−1).32 In contrast, a water solution of Triton x-45 at its 1 × CMC has dynamic surface tension comparable to that of water.32 This may directly contribute to an increase in the concentration of positive ions of studied metals in the near-cathode zone of the discharge available for the recombination (through a three-body collision involving one positive metal ion and two low energy electrons13,33) and the excitation of their atoms (by a collision with a high energy electron13,33), and so reflect the amplification of net intensities of metals observed following the addition of Triton x-405 to electrolyte solutions. On the other hand, by increasing the viscosity of electrolyte solutions,34 a lowering of the vaporization rate of H2O35,36 may occur and, as a consequence, a lower concentration of the H2O vapor in the core of the discharge results. Since, by nature, dc-APGD is operated in an environment saturated with the H2O vapor and which contains OH and H radicals as well as free electrons,6,12,13 any alteration of the concentration of the H2O vapor in the core of the discharge will certainly affect the concentration of the latter species. In this particular case, the increase in viscosity of electrolyte solutions after the addition of surfactants results in a decrease in the concentration of OH and H species and an increase in the concentration of free electrons in the discharge. Since heavier Triton x-405 is likely to increase the viscosity of electrolyte solutions to a greater extent than lighter Triton x-45,34 changes in the intensity of different species (decreases for molecular bands and atomic lines of H and O or increases for atomic lines of metals) observed in the emission spectrum of the discharge are clearly greater for the former surfactant.
3.3 Effect of the addition of surfactants on spectroscopic parameters
To elucidate elementary processes in the near-cathode zone of the discharge and check whether a change of rheological properties of electrolyte solutions results in changes in the concentration of discharge active species, selected spectroscopic parameters, i.e., Trot(OH), Trot(N2), Texc(Co I), Texc(H), ne and IMg II/IMg I, were determined.Fig. 3a illustrates the dependency of Trot(OH) and Trot(N2) on the concentration of Triton x-45 and Triton x-405 in the electrolyte solution. As can be seen, Trot(OH) decreases with the concentration of both surfactants which is likely to be due to an increase in the viscosity of electrolyte solutions, a lowering of the vaporization rate of H2O and a consequent reduction of the concentration of the H2O vapor and OH radicals, predominantly result from the dissociation of H2O molecules and the dissociative recombination of H2O+ ions,27,33,37 in the near-cathode zone. A greater 20% decrease was noted for the heavier surfactant, which expectedly increases the viscosity of electrolyte solutions to a greater extent. The behavior of Trot(OH) was consistent with the observed decrease in the intensity of the band head of the (0-0) OH A2Σ+→X2Π system. Changes of Trot(N2), noted with a stepwise addition of both surfactants, were however established not to be so significant, mostly because N2 diffuses from the surrounding air into the core of the discharge.21,27 Considering the error of the temperature estimation, Trot(N2), being about half of Trot(OH), was found to be practically unchanged when Triton x-45 was present in electrolyte solutions. For the addition of Triton x-405, a slow but steady decrease (by 12 ± 2%, on average) in Trot(N2) was observed when the concentration of the surfactant was changed from its 1 × CMC to 5 × CMC.
 | ||
| Fig. 3 Effect of concentration of Triton x-45 (dotted line) and Triton x-405 (solid line) on (a) rotational temperatures of OH and N2 molecules, (b) excitation temperatures of Co and H atoms and (c) the electron number density. | ||
Since a lower saturation of the discharge atmosphere with the H2O vapor was presumed to result in an increase in the concentration of free electrons, ne was determined and it is also expected that the difference in its values will be related to surfactant size. Indeed, when compared to the value of 3.98 × 10+14 cm−3 determined in conditions without the surfactant in the electrolyte solution (see Fig. 3c), the addition of Triton x-405 was established to rapidly increase ne (by 68%) already at the concentration of the surfactant corresponding to its ¼ × CMC. Then, values of ne were found to remain at the level 6.63 ± 0.17 × 10+14 cm−3, on average, regardless of the concentration of Triton x-405 in the electrolyte solution. When Triton x-45 was added, a slower and about two times smaller, when compared to Triton x-405, increase in ne was observed. Accordingly, a maximal 33% gain of ne was noted at the concentration of the surfactant corresponding to its 1 × CMC. Above this concentration, ne remained at the level of 5.10 ± 0.24 × 10+14 cm−3, on average. The release of an additional pool of free electrons in the discharge was found to be reflected by an increase in values of IMg II/IMg I, which varied from 0.037 (no surfactant present in the electrolyte solution) to maximally 0.055 (in the presence of Triton x-45 at its 5 × CMC) and 0.046 (in the presence of Triton x-405 at its 5 × CMC).
The enhancement of ne and the concentration of sputtered metal ions in the near-cathode zone of the discharge, as a result of the addition of surfactants to electrolyte solutions, is higher in case of Triton x-405 than for Triton x-45, which certainly implies respective amplifications of the net intensity of atomic emission lines of studied metals. This assumption is reflected by changes of Texc(Co I) and Texc(H) (see Fig. 3b). Accordingly, Texc(Co I) is established to increase from the value of 7000 K (no surfactant present in the solution) to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 K when Triton x-405 was added to the electrolyte solution at a concentration corresponding to its ¼ × CMC. A further increase in the concentration of the surfactant to its 5 × CMC resulted only in a slight increase of the temperature (not more than 600 K). A much slower increase in Texc(Co I) with increasing concentration of the surfactant in the electrolyte solution was observed for the lighter surfactant Triton x-45. This was increased to about 8600 K at the concentration of the surfactant corresponding to its 1 × CMC and, at higher concentrations, attained a plateau of about 8700 K. The error of the temperature estimation, Texc(H) remained in these unchanged conditions. This suggests that, despite a probable reduction of the concentration of H radicals in the near-cathode zone of the discharge due to the less intensive evaporation of H2O, an efficient excitation of H atoms was possible due to the relatively high energies of electrons released.
400 K when Triton x-405 was added to the electrolyte solution at a concentration corresponding to its ¼ × CMC. A further increase in the concentration of the surfactant to its 5 × CMC resulted only in a slight increase of the temperature (not more than 600 K). A much slower increase in Texc(Co I) with increasing concentration of the surfactant in the electrolyte solution was observed for the lighter surfactant Triton x-45. This was increased to about 8600 K at the concentration of the surfactant corresponding to its 1 × CMC and, at higher concentrations, attained a plateau of about 8700 K. The error of the temperature estimation, Texc(H) remained in these unchanged conditions. This suggests that, despite a probable reduction of the concentration of H radicals in the near-cathode zone of the discharge due to the less intensive evaporation of H2O, an efficient excitation of H atoms was possible due to the relatively high energies of electrons released.
Interestingly, it seems that the highest amplification of the net intensity of atomic emission lines of metals studied was achieved for those lines, for which excitation energies were within 3.7–4.9 eV (see Fig. 4). This preference was especially evident for different atomic emission lines of the same metal, where it can be expected that the enhancement of the net intensity due to the addition of the surfactant to the electrolyte solution and a higher sputtering rate of its surface will be the same for all lines. However, for atomic emission lines of Co at 240.7, 344.4, 358.7 and 359.5 nm, having excitation energies of 5.15, 4.11, 4.50 and 3.62 eV, respectively, the corresponding amplification factors determined were different, i.e., 3.4, 4.9, 4.8 and 3.0. A similar effect was observed for Ni I lines. Coincidentally, the excitation energy of the (0-0) band of the OH A2Σ+→X2Π system, for which a quenching of the intensity with the concentration of surfactant in the electrolyte solution was observed, falls at 4.1 eV.
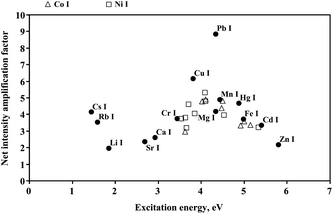 | ||
| Fig. 4 Amplification of the net intensity of atomic emission lines of different metals versus their excitation energies. Results obtained for Triton x-405 added to the electrolyte solution at the concentration corresponding to its 5 × CMC. Triangles: Co I lines at 240.7 nm (5.15 eV), 252.1 nm (4.92 nm), 252.9 nm (5.00 eV), 344.4 nm (4.11 eV), 345.4 nm (4.02 eV), 348.9 nm (4.47 eV), 358.7 nm (4.50 eV) and 359.5 nm (3.62 eV). Squares: Ni I lines at 232.0 nm (5.34 eV), 301.2 nm (4.54 eV), 303.8 nm (4.11 eV), 338.1 nm (4.09 eV), 344.6 nm (3.71 eV), 349.3 nm (3.66 eV), 351.5 nm (3.64 eV), 361.0 nm (3.54 eV) and 361.9 nm (3.85 eV). For other atomic emission lines, wavelengths and excitation energies are given in Table 1. | ||
The behavior of alkali metals (Li, Rb and Cs) producing monovalent ions was exceptional. Relatively high amplification factors of net intensities of Li 670.8 nm (the excitation energy of 1.85 eV, the ionization energy of 5.39 eV), Rb 780.0 nm (1.59 eV, 4.18 eV) and Cs 852.1 nm (1.45 eV, 3.89 eV) lines were obtained, i.e., 2.0, 3.5 and 4.2, respectively, although excitation energies for these lines are below 2 eV. This may be related to a higher contribution of recombination processes over the ionization for these metals in conditions of the addition of the surfactant. It is possible that this may be the result of a longer residence time of heavier ions in the near-cathode zone of the discharge and a higher probability of the recombination of Cs+ ions per unit of time when compared to Li+ ions.
4 Conclusions
It was found that the presence of non-ionic surfactants in electrolyte solutions that served as the liquid cathode in dc-APGD is responsible for substantial changes in the emission characteristic of this excitation source. Apparently, the addition of these organic compounds to electrolyte solutions results in an increase in their viscosity and a decrease of the dynamic surface tension. This is likely to lead to an increase in the sputtering rate of dissolved components of the solution (metal ions) and a decrease in the vaporization rate of water, particularly for Triton x-405. In consequence, when the near-cathode zone of the discharge is less saturated with water vapor molecules, the electron number density is enhanced, while the net intensity of atomic emission lines of all metals is improved by 4 to 9 times at a concentration corresponding to its 5 × CMC. When taking into account that the fluctuation of the background in the vicinity of these lines also decreases, it bodes for a very successful improvement to the analytical performance of the system. All the observed effects are likely to be related to the size of the surfactant, however, this requires further study.Acknowledgements
The work was financed by a statutory activity subsidy from Polish Ministry of Science and Higher Education for the Faculty of Chemistry of Wroclaw University of Technology.References
- M. R. Webb, F. J. Andrade and G. M. Hieftje, J. Anal. At. Spectrom., 2007, 22, 766–774 RSC.
- M. R. Webb, F. J. Andrade and G. M. Hieftje, Anal. Chem., 2007, 79, 7807–7812 CrossRef CAS.
- P. Jamroz, P. Pohl and W. Zyrnicki, J. Anal. At. Spectrom., 2012, 27, 1032–1037 RSC.
- M. R. Webb, F. J. Andrade, G. Gamez, R. McCrindle and G. M. Hieftje, J. Anal. At. Spectrom., 2005, 20, 1218–1225 RSC.
- T. Cserfalvi and P. Mezei, J. Anal. At. Spectrom., 2003, 18, 596–602 RSC.
- M. R. Webb, F. J. Andrade and G. M. Hieftje, Anal. Chem., 2007, 79, 7899–7905 CrossRef CAS.
- H. J. Kim, J. H. Lee, M. Y. Kim, T. Cserfalvi and P. Mezei, Spectrochim. Acta, Part B, 2000, 55, 823–831 CrossRef.
- P. Mezei, T. Cserfalvi, M. Janossy, K. Szocs and H. J. Kim, J. Phys. D: Appl. Phys., 1998, 31, 2818–2825 CrossRef CAS.
- M. R. Webb and G. M. Hieftje, Anal. Chem., 2009, 81, 862–867 CrossRef CAS.
- M. A. Mottaleb, Y. A. Woo and H. J. Kim, Microchem. J., 2001, 69, 219–230 CrossRef CAS.
- Y. S. Park, S. H. Ku, S. H. Hong and H. J. Kim, Spectrochim. Acta, Part B, 1998, 53, 1167–1179 CrossRef.
- M. A. Mottaleb, J. S. Yang and H. J. Kim, Appl. Spectrosc. Rev., 2002, 37, 247–273 CrossRef CAS.
- P. Mezei and T. Cserfalvi, Appl. Spectrosc. Rev., 2007, 42, 573–604 CrossRef CAS.
- M. R. Webb, G. C. Y. Chan, F. J. Andrade, G. Gamez and G. M. Hieftje, J. Anal. At. Spectrom., 2001, 21, 525–530 RSC.
- P. Mezei and T. Cserfalvi, J. Phys. D: Appl. Phys., 2006, 39, 2534–2539 CrossRef CAS.
- P. Mezei, T. Cserfalvi and L. Csillag, J. Phys. D: Appl. Phys., 2005, 38, 2804–2811 CrossRef CAS.
- A. Shaltout, Microchim. Acta, 2006, 155, 447–452 CrossRef CAS.
- R. Shekhar, D. Karunasagar, M. Ranjit and J. Arunachalam, Anal. Chem., 2009, 81, 8157–8166 CrossRef CAS.
- R. Shekhar, D. Karunasagar, K. Dash and M. Ranjit, J. Anal. At. Spectrom., 2010, 25, 875–879 RSC.
- R. Shekhar, Talanta, 2012, 93, 32–36 CrossRef CAS.
- P. Jamroz and W. Zyrnicki, Plasma Chem. Plasma Process., 2011, 31, 681–696 CrossRef CAS.
- Z. Zhu, G. C. Y. Chan, S. J. Ray, X. Zhang and G. M. Hieftje, Anal. Chem., 2008, 80, 7043–7050 CrossRef CAS.
- A. Sanz-Medel, M. del Rosario Fernandez de la Campa, E. Blanco Gonzalez and M. L. Fernandez-Sanchez, Spectrochim. Acta, Part B, 1999, 54, 251–287 CrossRef.
- S. K. Hait and S. P. Moulik, J. Surfactants Deterg., 2001, 4, 303–309 CrossRef CAS.
- J. Luque and D. R. Crosley, Lifbase: Database and Spectral Simulation (Version 1.5), SRI International Report MP 99-009, 1999 Search PubMed.
- C. O. Laux, Radiation and Nonequilibrium Collisional-Radiative Models, von Karman Institute for Fluid Dynamics, Lecture Series 2002-07, Rhode Saint-Genese, Belgium, 2002 Search PubMed.
- P. Jamroz, W. Zyrnicki and P. Pohl, Spectrochim. Acta, Part B, 2012, 73, 26–34 CrossRef CAS.
- NIST Atomic Spectra Database, www.physics.nist.gov/PhysRefData/ASD/index.html.
- M. A. Gigosos, M. A. Gonzalez and V. Cardenoso, Spectrochim. Acta, Part B, 2003, 58, 1489–1504 CrossRef.
- R. W. B. Pearse and A. G. Gaydon, The Identification of Molecular Spectra, Chapman & Hall, London, 1976 Search PubMed.
- J. Perkowski, J. Mayer and L. Kos, Fibres Text. East. Eur., 2005, 13, 81–85 CAS.
- V. B. Fainerman, A. V. Mys, E. V. Aksenenko, A. V. Makievski, J. T. Petkov, J. Yorke and R. Miller, Colloids Surf., A, 2009, 334, 22–27 CrossRef CAS.
- P. Mezei and T. Cserfalvi, Sensors, 2012, 12, 6576–6586 CrossRef CAS.
- L. Qiao and A. J. Easteal, Colloid Polym. Sci., 1996, 274, 974–980 CAS.
- A. Frohn and N. Roth, Dynamics of Droplets, Springer Verlag, Berlin, 2000 Search PubMed.
- A. Faghri and Y. Zhang, Transport Phenomena in Multiphase Systems, Elsevier, Amsterdam, 2006 Search PubMed.
- P. Mezei, T. Cserfalvi, P. Hartmann and L. Bencs, Spectrochim. Acta, Part B, 2010, 65, 218–224 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
