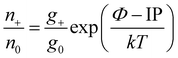Evaluation of colloidal silicagels for lead isotopic measurements using thermal ionisation mass spectrometry†
Magdalena H.
Huyskens
*,
Tsuyoshi
Iizuka‡
and
Yuri
Amelin
The Australian National University, Research School of Earth Sciences, 61 Mills Road, 0200 Acton, Canberra, ACT, Australia. E-mail: magda.huyskens@anu.edu.au; yuri.amelin@anu.edu.au; Fax: +6126125 0941; Tel: +61261254835
First published on 30th May 2012
Abstract
In this study we have investigated the suitability of silicagels from four companies (Merck, Sigma-Aldrich, Nissan Chemical and Alfa Aesar) as emission activators for ionisation of lead in thermal ionisation mass spectrometry (TIMS). We have tested U and Pb blank levels, ionisation efficiency, signal stability, degree of fractionation, and reproducibility and accuracy of the Pb isotopic ratios measured with these emitters. Further tests were undertaken to evaluate the dependency of ionisation efficiency on the particle size and concentration of silicagels and the mixing ratio of silicagel and phosphoric acid. Mass fractionation, reproducibility and stability of the ion beam were monitored using 300 pg of the lead isotopic standard SRM-981. The Merck and Sigma-Aldrich silicagels have the best performance as Pb ion emitters. They yielded accurate Pb isotopic ratios with precisions of ∼0.05% (2SD) for 207Pb/206Pb. We find that the ionisation efficiency does not depend on the particle size, but on silicagel concentration and proportion of silicagel to phosphoric acid. The highest ionisation efficiency (9.2 ± 2.2%, 2SE) was obtained using 0.004 ml of a silicagel from Sigma-Aldrich at a concentration of 0.4 wt% of SiO2 loaded with 0.05 ml of 0.02 N phosphoric acid. These results clearly indicate that the Sigma-Aldrich emitter is an excellent alternative for Pb isotopic measurements to the widely used, but no longer produced Merck silicagel. The silicagels from Alfa Aesar and Nissan Chemical have a high U background concentration compared to the Merck and Sigma-Aldrich gels, and produce less accurate Pb isotopic ratios.
Introduction
Precise determination of Pb isotopic composition is crucial in several disciplines e.g. ore formation studies, environmental studies and U–Pb geochronology. Several analytical techniques are used to determine Pb isotopic compositions: inductively coupled plasma mass spectrometry, secondary ion mass spectrometry and thermal ionisation mass spectrometry (TIMS). Since the analytical precision is directly dependent on the number of ions detected in the mass spectrometers, efficient emission of ions is a prerequisite for analysis of the small quantities of Pb that are usually available in geochronological and environmental research. In the case of TIMS Pb isotopic analysis, ionisation efficiency can be significantly improved by loading samples with silicagel. The first report on using silicagel as an ionisation activator for Pb isotopic analysis was by Akishin et al.,1 in which a zirconia–silica emitter was produced by mixing solutions of sodium silicate and zirconium sulphate and cleaned with distilled water and phosphoric acid. Cameron et al.2 followed this approach, but found it difficult to prepare a sufficiently clean gel using this technique. They found that silicagel without added zirconia was easier to prepare, and it was as effective as the mixture with zirconia. The silicagel was prepared by dissolving sodium metasilicate in water and nitric acid, and phosphoric acid was added during the loading procedure. Gerstenberger and Haase3 improved the silicagel emitter further in describing a simple and low contamination procedure to prepare silicagel. For their method a colloidal silicic acid produced by the chemical company Merck (Art. no. 12475) is added drop by drop to 0.1 N phosphoric acid during agitation. Currently, this colloidal silicic acid is the most effective and widely used emitter for Pb isotopic measurements, but is no longer available for purchase. Another ion emitter will be needed if the emitter efficiency of colloidal silicic acid deteriorates with time, or the available stocks are used up. More recent studies focussed on improving ionisation efficiency in two different ways. Miyazaki et al.4 focussed on producing a silicagel with a small particle size, since this was suggested to increase the ionisation efficiency.3 Nohda et al.5 searched for supplementary materials to increase the signal intensity and prolong the lifetime of the ion beam. Ge and Re were added to colloidal silicagel, which enhanced the ion beam for samples larger than 2.5 ng of Pb. None of these studies however, succeeded in producing a silicagel that would surpass the efficiency of the emitter of Gerstenberger and Haase.3 In addition to high efficiency of Pb ionisation, a good emitter must produce a stable ion beam (necessary for peak jumping measurements with ion counting multipliers), provide reproducible running conditions and mass fractionation patterns, and have low background concentration of Pb. An additional requirement for emitter use in high-precision U–Pb geochronology is the ability to produce long-lasting (2–4 hours) ion beams for both Pb and U, and with a low background concentration of uranium.In this study, we present the tests of currently commercially available colloidal silicagels from three major manufacturers: Sigma-Aldrich, Nissan Chemical and Alfa Aesar, as possible alternatives to the established Merck emitter. The gels were evaluated for U and Pb blanks, ion yield (number of ions detected divided by number of atoms loaded), reproducibility and accuracy of resultant Pb isotopic ratios for the Pb isotopic standard SRM-981. We also checked the dependencies of ion yield on the particle size and concentration of silicagel and on the mixing proportion of silicagel and H3PO4. We use these results to briefly discuss the possible mechanisms of ionisation enhancement, but detailed assessment of the physical and chemical background of Pb ion emission from molten silica is outside the scope of this study.
Materials
Prior to the achievements of Gerstenberger and Haase3 silicagel was produced by dissolving sodium metasilicate in water and adding nitric acid,2 or by the hydrolysis of silicon tetrachloride.6,7 Commercial colloidal silica is commonly produced by the sol–gel technique using tetraethylorthosilicate (TEOS, Si(OC2H5)4) (e.g. Klein8). By mixing TEOS with water and a solvent (usually ethanol) using either an acid or a base as catalyst, colloidal silica is produced. Acid catalysed reactions tend to form linear polymers, whereas base catalysed silicagels tend to form branched clusters (e.g. Klein8). Therefore particle size tends to be smaller in base catalysed sol–gel preparation of silicagel.Silicagels from the companies Sigma-Aldrich, Nissan Chemical and Alfa Aesar were chosen for this study and compared to the established Merck silicagel.3 The properties of the silicagels are summarised as follows:
(I) The Merck silicic acid (article no. 12475, similar to the material described by Gerstenberger and Haase3) has a concentration of ∼11 wt% of SiO2 and contains 15% methanol. Unfortunately no particle size is reported.
(II) The silicagel from Sigma-Aldrich (article no: 701491) has an original concentration of 20 wt% SiO2 and is dispersed in H2O. The particles have a size of <50 nm and it contains 7.5 wt% of aluminium (as Al2O3) and 0.025 wt% of ammonium hydroxide.
(III) Nissan Chemical provides silicagels with variable particle size, allowing us to investigate a possible correlation of ionisation efficiency and particle size of SiO2. The investigated materials were Organosilicasol™ IPA-ST, IPA-ST-L and IPA-ST-ZL with particle sizes of 10–15 nm, 40–50 nm and 70–100 nm, respectively. The concentration of SiO2 is 31 wt% and isopropanol is used as a solvent.
(IV) The colloidal silicagel of Alfa Aesar (article no: 36669) has a concentration of 15% and the 4 nm SiO2 particles are in dispersion with H2O.
Methods
The concentrated gels were diluted with water to achieve concentrations of 0.1 to 2 wt% of SiO2 and homogenised for 30 minutes in an ultrasonic bath. The Merck silicagel was prepared after the recipe of Gerstenberger and Haase.3 For most of the measurements an aliquot of the NIST SRM-981 standard containing 300 pg of Pb was spiked with 30 pg of a 202Pb–205Pb mixed tracer described by Amelin and Davis9 and dried together with 0.08 ml of 0.02 N H3PO4. Varying amounts of 0.02 N H3PO4 (between ∼0.02 and 0.16 ml) were used with the 0.4% silicagel of Sigma-Aldrich to determine the most effective mixing proportion. Before loading on to outgassed zone refined Re-filaments (99.999% Re, 0.076(7) cm wide, 0.0030(3) cm thick, H. Cross Company), the silicagels were shaken and an amount of approximately 0.004 ml of gel was used for loading the standard. The filament current was slowly increased (in ∼60 s) to 1.9 A and maintained at this current for 60 s. Then the current was increased slowly until phosphoric acid was fumed away and then the current was immediately turned down. The Finnigan MAT 261 TIMS at the Australian National University (ANU) was used for the analyses. Pb isotopes were measured simultaneously on Faraday cups except for blank measurements, which were performed on a secondary electron multiplier. Isotopic ratio determinations were based on 8–24 blocks of measurements, depending on the timing of sample exhaustion, and each block consists of 10 cycles of 16 s integration time. Prior to the measurements, the filament temperature was raised continuously to 1.6 A in 5 minutes, then to 1.9 A in approximately 5–10 minutes. During the measurements, the current was increased after each block by 0.03–0.05 A, until a steady or slowly decreasing ion beam was established. After that, the filament current (usually about 2.15–2.30 A) remained unchanged until the sample load is exhausted. The interference of BaPO2+ with 202Pb was monitored and corrected by measuring mass 201. The mass fractionation factor was obtained using the measured 202Pb/205Pb ratio and a power law correction was applied. The mean corrected values for each sample were calculated using Isoplot/Ex version 3.00.10 The final isotopic ratios were obtained after correction for blanks, instrumental mass fractionation, spike and associated errors using the PBDAT software.11To investigate possible causes of the differences in ionisation efficiency, we have studied the structure of fused silicagel loads using electron microscope imaging. The samples were loaded onto a Re filament as previously described, with the silicagels Sigma-Aldrich (0.4%), Nissan Chemical IPA-ST (0.4%) and Merck (0.1%). The filaments were placed in a filament outgasser. After a high vacuum had been achieved (<100 μPa), the filaments were heated slowly, simulating heating in the mass spectrometer, and kept at a current of 1.9 A for 30 min to simulate the beginning of the measurement. Another set of loaded filaments was heated to 2.2 A and kept there for 30 min to simulate the end of a measurement. The filaments were then stripped off the posts, preserving the sample loads, and put onto carbon tape for secondary electron (SE) imaging with the scanning electron microscope Jeol JSM-6610A. Along with SEM imaging, we also took spot analyses using energy-dispersive X-ray spectroscopy (EDS) to estimate the composition of the phases observed on the filament.
Results
The measured blank levels for the silicagels are summarised in Table 1. The Merck silicagel gave the lowest blanks for Pb and U. The Sigma-Aldrich silicagel yielded a U blank comparable to the Merck silicagel, but a slightly higher Pb blank. The blanks of the Alfa Aesar silicagel are substantially higher than those of the Merck silicagel for both Pb and U. For the silicagels from Nissan Chemical, extremely high U blanks were observed.| Silicagel | Conc. [%] | 204Pb/206Pb | 2SD | 207Pb/206Pb | 2SD | 208Pb/206Pb | 2SD | Average ion yield [%] | 2SE | n |
|---|---|---|---|---|---|---|---|---|---|---|
| a NC: Nissan Chemical; SD: standard deviation; SE: standard error; n: number of measurements. | ||||||||||
| Merck | 0.1 | 0.05903 | 0.00002 | 0.91474 | 0.00004 | 2.16789 | 0.00008 | 4.2 | 0.5 | 25 |
| Merck | 0.4 | 0.05904 | 0.00003 | 0.91464 | 0.00008 | 2.16809 | 0.00039 | 2.6 | 0.4 | 13 |
| Sigma-Aldrich | 0.1 | 0.05898 | 0.00003 | 0.91471 | 0.00008 | 2.16785 | 0.00023 | 2.9 | 3.1 | 2 |
| Sigma-Aldrich | 0.15 | 0.05906 | 0.00037 | 0.91474 | 0.00005 | 2.16785 | 0.00018 | 4.0 | 2.2 | 2 |
| Sigma-Aldrich | 0.2 | 0.05904 | 0.00002 | 0.91478 | 0.00012 | 2.16808 | 0.00521 | 4.2 | 1.1 | 2 |
| Sigma-Aldrich | 0.3 | 0.05902 | 0.00008 | 0.91475 | 0.00004 | 2.16765 | 0.00044 | 5.1 | 1.4 | 4 |
| Sigma-Aldrich | 0.4 | 0.05904 | 0.00001 | 0.91473 | 0.00003 | 2.16786 | 0.00006 | 6.1 | 1.0 | 21 |
| Sigma-Aldrich | 0.5 | 0.05901 | 0.00002 | 0.91448 | 0.00088 | 2.16760 | 0.00298 | 5.5 | 0.8 | 2 |
| Sigma-Aldrich | 0.7 | 0.05909 | 0.00020 | 0.91465 | 0.00006 | 2.16783 | 0.00018 | 4.9 | 2.5 | 3 |
| Sigma-Aldrich | 1 | 0.05903 | 0.00004 | 0.91463 | 0.00127 | 2.16762 | 0.00299 | 6.4 | 0.9 | 2 |
| NC IPA-ST | 0.2 | 0.05896 | 0.00003 | 0.91432 | 0.00010 | 2.16701 | 0.00033 | 2.8 | 0.9 | 2 |
| NC IPA-ST | 0.4 | 0.05904 | 0.00037 | 0.91472 | 0.00008 | 2.16785 | 0.00020 | 4.0 | 1.8 | 2 |
| NC IPA-ST | 0.6 | 0.05893 | 0.00162 | 0.91448 | 0.00008 | 2.16784 | 0.00021 | 3 | 2.1 | 2 |
| NC IPA-ST-L | 0.2 | 0.05904 | 0.00003 | 0.91452 | 0.00011 | 2.16749 | 0.00025 | 3.9 | 1.3 | 2 |
| NC IPA-ST-L | 0.4 | 0.05896 | 0.00015 | 0.91446 | 0.00070 | 2.16767 | 0.00143 | 2.1 | 1.6 | 3 |
| NC IPA-ST-L | 0.6 | 0.05898 | 0.0002 | 0.91385 | 0.00018 | 2.16644 | 0.00061 | 1.6 | — | 1 |
| NC IPA-ST-ZL | 0.2 | 0.05901 | 0.00003 | 0.91451 | 0.00138 | 2.16737 | 0.00026 | 4.4 | 0.7 | 2 |
| NC IPA-ST-ZL | 0.4 | 0.05902 | 0.00003 | 0.91456 | 0.00036 | 2.16764 | 0.00120 | 3.5 | 0.4 | 3 |
| NC IPA-ST-ZL | 0.6 | 0.05909 | 0.00004 | 0.91446 | 0.00012 | 2.16727 | 0.00459 | 2.3 | 2.4 | 2 |
| Alfa Aesar | 0.1 | 0.05897 | 0.00011 | 0.91482 | 0.00014 | 2.16818 | 0.00068 | 2.7 | — | 1 |
| Alfa Aesar | 15 | 0.05905 | 0.00010 | 0.91502 | 0.00037 | 2.16816 | 0.00113 | 1.6 | — | 1 |
We measured 107 loads of the NIST standard SRM-981. Isotopic data were examined for consistency over the whole analysis time, considering that degrees of fractionation and isobaric interferences change with time. Examples of within run variations of the ion beam intensity, fractionation factor, fractionation corrected 204Pb/206Pb and 207Pb/206Pb ratios, measured 201(BaPO4)/206Pb ratio and 207Pb/206Pb ratio normalised to the 208Pb/206Pb ratio are given in Fig. 1. The average fractionation factor for the Merck 0.1% silicagel is 1.00092 ± 0.00024 (2SD). Measurements with an extremely high or low fractionation factor for 202Pb/205Pb (below 1 or above 1.0015) or with a high BaPO2+ content (weighted mean of the measured 201(BaPO4)/206Pb ratio above 0.01; usual ratio is ∼0.003) were excluded, which resulted in the elimination of 10 of the 107 loads measured. Most of the measurements using the Merck 0.4% silicagel and the Nissan Chemical silicagels showed high signals of BaPO2+ at high temperatures (Fig. 1). Since this affected all measurements with these silicagels, only measurements with extremely high or low fractionation factors were excluded from the summary. Four measurements out of the total of 29 with the Merck 0.1% silicagel emitter were excluded due to high BaPO2+ signals. For measurements with the Sigma-Aldrich silicagels four out of 42 measurements were excluded. Two showed a high BaPO2+ signal, one a high fractionation factor and one load flaked. The ionisation efficiency of the established Merck silicagel is compared to colloidal silicagels from Sigma-Aldrich, Nissan Chemical and Alfa Aesar (Fig. 2a). The average ion yield for all investigated silicagels is summarised in Table 2. The full data table can be found in the ESI†.
 | ||
| Fig. 1 Within run variations of the ion beam intensity, fractionation factor, fractionation corrected 204Pb/206Pb and 207Pb/206Pb ratios, measured 201/206 ratio and 207Pb/206Pb ratio normalised to the 208Pb/206Pb ratio of the Merck 0.1%, Sigma-Aldrich 0.4%, Nissan Chemical IPA-ST 0.2% and Alfa Aesar 0.1% silicagels. Error bars are 2SD. | ||
 | ||
| Fig. 2 (a) Ion yield versus the concentration of all investigated silicagels. (b) Dependency of the ion yield of the 0.4% Sigma-Aldrich silicagel on the amount of phosphoric acid. | ||
| Conc. [%] | Pb [pg] | U [pg] | |
|---|---|---|---|
| Merck | 0.1 | 0.03 | 0.06 |
| Sigma-Aldrich | 0.4 | 0.29 | 0.11 |
| Sigma-Aldrich | 1 | 0.32 | 0.07 |
| Nissan Chemical IPA-ST | 0.4 | 0.16 | 21.5 |
| Nissan Chemical IPA-ST-L | 0.4 | 0.46 | 30.4 |
| Nissan Chemical IPA-ST-ZL | 0.4 | 0.32 | 37.9 |
| Alfa Aesar | 0.1 | 0.72 | 0.72 |
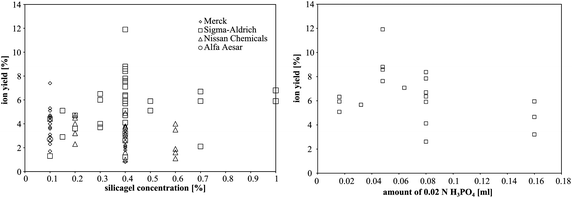
The average ion yield (%) for the established Merck emitter gel is 4.2 ± 0.5 (2SE, n = 25). For the silicagel from Sigma-Aldrich the ion yield increased from 2.9 ± 3.1 to 6.1 ± 1.0 with increasing SiO2 concentration from 0.1 to 0.4%. For the concentrations between 0.4 and 1%, the ion yield is constant at ∼6% (Fig. 3). The signal lifetime was prolonged, but the maximum intensity decreased with increasing concentration of SiO2. For the silicagels from Nissan Chemical, however, no correlation between ion yield and silicagel concentration (0.2%, 0.4% and 0.6%) is observed. Furthermore, three Nissan Chemical silicagels with different particle sizes gave similar ion yields: the average ion yield for Nissan Chemical ST is 3.2 ± 0.9, for Nissan Chemical ST-L 2.6 ± 1.0 and for Nissan Chemical ST-ZL 3.4 ± 0.9. We obtained ion yields of 2.9 and 1.6 using Alfa Aesar silicagel with concentrations of 0.1% and 1.5%, respectively.
 | ||
| Fig. 3 Average ion yield of the Sigma-Aldrich silicagel for different concentrations. Error bars are 2SE. | ||
The effect of the amount of H3PO4 on the ion yield was tested using the Sigma-Aldrich silicagel with the concentration of 0.4%. The results are shown in Fig. 2b and Table 3. The ion yield changed with the amount of phosphoric acid and the highest average ion yield of 9.2 ± 2.2 is obtained with an amount of 0.05 ml of H3PO4.
| H3PO4 [ml] | Average ion yield [%] | 2SE | n |
|---|---|---|---|
| 0.02 | 5.78 | 0.74 | 3 |
| 0.03 | 5.67 | — | 1 |
| 0.05 | 9.23 | 2.15 | 4 |
| 0.06 | 7.07 | — | 1 |
| 0.08 | 5.99 | 1.53 | 7 |
| 0.16 | 4.61 | 1.58 | 3 |
All obtained isotopic ratios were compared to the values of SRM-981 obtained by Amelin12 with the Merck 0.1% silicagel and a TRITON TIMS at the Geological Survey of Canada. All isotopic ratios determined with the Merck 0.1% silicagel agree with these values, as do all, with one exception, obtained with the Sigma-Aldrich silicagels. For this one measurement the 207Pb/206Pb ratio disagrees by 0.00004. The average isotopic ratios and reproducibility for the Merck and Sigma-Aldrich silicagel are comparable (e.g.207Pb/206Pb = 0.91472 ± 0.00002 for Sigma-Aldrich and 207Pb/206Pb = 0.91474 ± 0.00004 for Merck). The Nissan Chemical silicagels gave consistent 204Pb/206Pb ratios, but the 208Pb/206Pb and 207Pb/206Pb ratios did result in lower values, with the 208Pb/206Pb ratio just within error of the reported values. The 207Pb/206Pb ratio yielded a mean value of 0.91454 ± 0.00008. The preliminary data of the Alfa Aesar silicagel indicates that the Pb isotopic ratios agree with the reported values.12
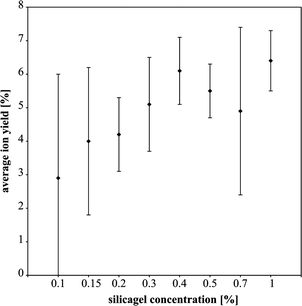
In order to check for possible differences in texture of the studied gels, SE images of loaded filaments were taken (Fig. 4). In every case a glass was formed during the simulations. The SE images reveal that the gel layer consists of the glass and areas where the glass is exhausted and the Re filament is exposed (Fig. 4a–c and 5a and b). Where the glass is in direct contact with the Re filament, the glass can also contain Re (Fig. 5c). Notably, the glass of the Merck emitter mostly contains Re, whereas those of the other emitters do not. For the simulations of the end of TIMS measurements at higher temperatures, Re was incorporated into the glass to the same extent in all experiments. For the Nissan Chemical and Sigma-Aldrich studies dissolution structures of Re from the filament can be observed (Fig. 4d and e) and to a lesser extent also for Merck. The Merck silicagel appears to have more nucleation sites than the Nissan Chemical and Sigma-Aldrich silicagels.
 | ||
| Fig. 4 Secondary electron images of (a) Nissan Chemical, (b) Sigma-Aldrich and (c) Merck silicagels are shown of simulations at the beginning of the measurements. Black areas represent the glass originated from the silicagel. Light grey areas are Re from the filament and dark grey areas are glass that incorporated some Re from the filament. The white areas on image (e) are artefacts. Images (d), (e) and (f) show SE images for silicagels of Nissan Chemical, Sigma-Aldrich and Merck respectively for end of the measurements simulations. | ||
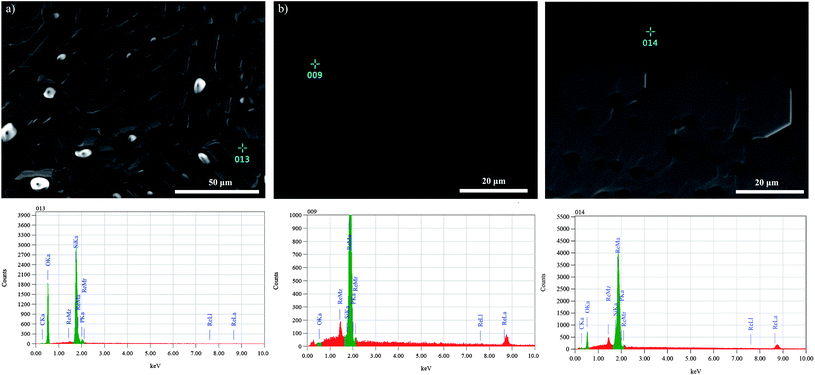 | ||
| Fig. 5 SE images and spot EDS analyses are shown. In image (a) the composition of the molten glass is shown. Except for silica it also contains phosphorus. Image (b) shows the Re of the filament and (c) shows that the glass can also incorporate Re. | ||
Discussion
Evaluation of the silicagels
Critical examination of the data quality is crucial for choosing the best emitter for Pb isotopic measurements. Therefore attention should be paid to the fractionation factor and pattern and the consistency of isotopic ratios over the temperature and time range of the analysis in order to identify isobaric interferences or mass-independent fractionation.12 In several measurements of this dataset, an isobaric interference of BaPO2+ with Pb was observed. This interference affects most significantly the mass 202, but also 204 and 205.9 A correction on mass 202 was applied for this interference and the contribution on the masses 204 and 205 were insignificant for all measurements. For most of the measurements that used the Merck 0.1% and Sigma-Aldrich silicagels, this isobaric interference was minor and an increase of the BaPO2+ ion beam was observed only at the end of a measurement. A slight increase of the amount of BaPO2+ was observed with increasing SiO2 concentration for the Sigma-Aldrich silicagel and a significant rise for the Merck silicagel at a SiO2 concentration of 0.4%. For the Nissan Chemical silicagels the BaPO2+ content was higher and the ion beam increased after a few blocks of the measurement. Nevertheless, after the applied interference corrections, the accuracy was not affected, with only slightly higher errors in the results (see discussion in Amelin and Davis9 for estimation of uncertainty involved with this correction). For the Nissan Chemical silicagel a major discrepancy in the isotopic ratios is observed in the 207Pb/206Pb ratio, but a shift to a lower ratio can also be observed in the 208Pb/206Pb ratio (although within error of the reported value12). The 207Pb/206Pb ratio can be affected by mass-independent fractionation,9 but no evidence for this effect was observed in the dataset. No explanation for the poor accuracy in this dataset could be found.Considering all aspects that characterise a good emitter for Pb, the Sigma-Aldrich silicagel is the best. It has higher ionisation efficiency, produces a long lasting stable ion beam, isotopic ratios that are accurate and reproducible and background levels of U and Pb are low. The silicagels of Nissan Chemical and Alfa Aesar are unsuitable emitters for Pb isotopic measurements due to high blank levels, mainly of U and a poor reproducibility and accuracy for the Nissan Chemical silicagels.
Effect of mixing proportion of silicagel and phosphoric acid on ionisation efficiency
The effect of the amount of phosphoric acid on the ion yield was tested using the 0.4% silicagel of Sigma-Aldrich. Aliquots of 0.004 ml of silicagel mixed 0.02 to 0.16 ml of 0.02 N H3PO4 define a normal distribution with a maximum ion yield for 0.05 ml of phosphoric acid. This mixing proportion might be the optimum for this silicagel, but may not be universally applicable to other concentrations or brands of silicagels.Effect of SiO2 particle size on ionisation efficiency
In order to evaluate the effect of the particle size on the ionisation efficiency, we studied the Nissan Chemical silicagels with varying particle size. Our results indicate no noticeable dependence of ionisation efficiency on silicagel particle size, inconsistent with previous studies.3,5,13 Note however that the previously suggested dependency is based on experiments using silicagels which vary not only in particle size, but also have different solvents and impurities. By contrast, our test was performed on a series of gels from the same company using the same solvent, allowing a more rigorous evaluation of the particle size effect. Indeed, Miyazaki et al.13 also analysed four silicagels with varying particle size between 34 and 122 nm from one company, and found no significant difference in the ion yield among them.Possible controls of ionisation efficiency
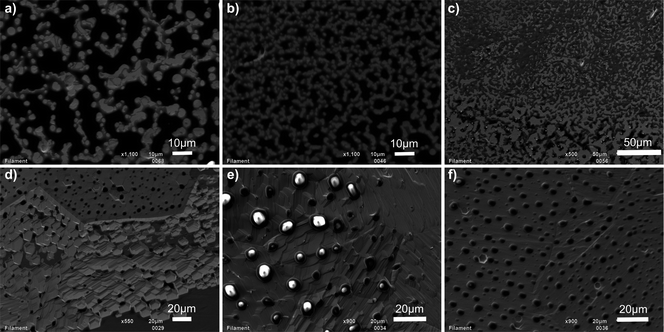
The process of thermal ion emission from silicagels and molten glasses in general is not fully understood. One proposed mechanism is that Pb in ionic form, which is loaded onto the filament, is reduced to neutral Pb by oxidizing Re.14 The ionization is thought to take place at the melt–vapour interface roughly following the Saha–Langmuir equation:where n+/n0 is the ion yield, g+ and g0 are the statistical weights of the ion and neutral states, Φ is the electron work function, IP the ionization potential of the element, k Boltzmann's constant and T the temperature.14
From this equation it appears that the ion yield mainly depends on the work function of the metal. Re itself has a work function for positive ion emission of ∼5 to 5.5 eV (compilation in Kawano15), which is too low for producing a detectable amount of ions in a mass spectrometer under conditions typical for silicagel runs. However, when Re gets oxidised the work function increases and can reach 7.2 eV.16 This is one possible explanation for the increase in the ion yield when silicagel is used, compared to direct surface ionisation in a double filament source. Experimental studies suggest that Re can be dissolved in silicate melts as an oxide species.17 Our observation of the presence of Re in the molten glass as seen in EDS further points towards this process.
An important difference among the three tested silicagels is that the most efficient one, from Sigma-Aldrich contains 7.5 wt% Al2O3. It is possible that either the Al2O3 stabilises Re oxides, or that they are bonding to Al on the surface.18–20 If alumina is stabilising Re oxide on the surface of the molten glass, then doping silicagel with alumina could enhance ionisation efficiency. Unfortunately an Al2O3-free version of the silicagel with the same properties (e.g. particle size and shape, concentration) from Sigma-Aldrich is not available to test the influence of alumina doping. Alumina is also used as an enhancer in other isotopic studies, e.g. tin, chromium, iron and zinc using a loading mixture of silicagel and boric acid.21–23
Conclusions
We have demonstrated that in terms of U and Pb blanks, Pb ionisation efficiency, accuracy and reproducibility of Pb isotopic ratio measurements the Sigma-Aldrich silicagel is superior to those from the Nissan Chemical and Alfa Aesar, and comparable to Merck silicagel. The only demerit of the Sigma-Aldrich silicagel compared to the one of Merck is the slightly higher blank for Pb, which may be problematic for samples with very low Pb content. The Alfa Aesar and Nissan Chemical silicagels are not suitable for high-precision U–Pb dating owing to the high U blank. We found that there is no clear correlation between particle size and ion yield in the range of 10–100 nm. However, a slight correlation between the amount of H3PO4 used during loading could be recognised. An amount of 0.05 ml of 0.02 N H3PO4 loaded with an amount of 0.004 ml of 0.4% silicagel was found to be the most effective mixture for the Sigma-Aldrich silicagel. Amounts of 0.05 to 0.08 ml of 0.02 N H3PO4 and concentrations of the silicagel ranging between 0.4 and 1% also resulted in high ionisation efficiency.Acknowledgements
We thank Nissan Chemical for supplying silicagels used in this study.References
- P. A. Akishin, O. T. Nikitin and G. M. Panchenkov, Geochemistry, 1957, 500–505 Search PubMed.
- A. E. Cameron, D. H. Smith and R. L. Walker, Anal. Chem., 1969, 41, 525–526 CrossRef CAS.
- H. Gerstenberger and G. Haase, Chem. Geol., 1997, 136, 309–312 CrossRef CAS.
- T. Miyazaki, T. Shibata and M. Yoshikawa, Proc. Jpn. Acad., Ser. B, 2003, 79, 58–62 CrossRef CAS.
- S. Nohda, B. S. Wang, C. F. You and R. Shinjo, Geochem. J., 2011, 45, 169–174 CAS.
- Y. Koide and E. Nakamura, Mass Spectrosc., 1990, 38, 241–252 CrossRef CAS.
- S. Nohda, Geochem. J., 1999, 33, 133–139 CrossRef CAS.
- L. C. Klein, Annu. Rev. Mater. Sci., 1985, 15, 227–248 CrossRef CAS.
- Y. Amelin and W. J. Davis, J. Anal. At. Spectrom., 2006, 21, 1053–1061, 10.1039/b606842a.
- K. R. Ludwig, Berkeley Geochronology Center Special Publication, 2003.
- K. R. Ludwig, U. S. Geol. Surv. Open-File Rep., 1993, 88–542, 1–33 Search PubMed.
- Y. Amelin, Geochim. Cosmochim. Acta, 2008, 72, 221–232 CrossRef CAS.
- T. Miyazaki, T. Shibata, M. Yoshikawa, T. Sakamoto, K. Iijima and Y. Tatsumi, Frontier Research on Earth Evolution, 2005, vol. 2, pp. 1–5 Search PubMed.
- G. F. Kessinger and J. E. Delmore, Int. J. Mass Spectrom., 2002, 213, 63–80 CrossRef CAS.
- H. Kawano, Prog. Surf. Sci., 2008, 83, 1–165 CrossRef CAS.
- W. D. Davis, Environ. Sci. Technol., 1977, 11, 587–592 CrossRef CAS.
- W. Ertel, H. S. O'Neill, P. J. Sylvester, D. B. Dingwell and B. Spettel, Geochim. Cosmochim. Acta, 2001, 65, 2161–2170 CrossRef CAS.
- J. C. Mol, Catal. Today, 1999, 51, 289–299 CrossRef CAS.
- F. C. Sheu, C. T. Hong, W. L. Hwang, C. J. Shih, J. C. Wu and C. T. Yeh, Catal. Lett., 1992, 14, 297–304 CrossRef CAS.
- M. Sibeijn, R. Spronk, J. A. R. Vanveen and J. C. Mol, Catal. Lett., 1991, 8, 201–208 CrossRef CAS.
- K. J. R. Rosman, R. D. Loss and J. R. Delaeter, Int. J. Mass Spectrom. Ion Processes, 1984, 56, 281–291 CrossRef CAS.
- A. Trinquier, J. L. Birck and C. J. Allegre, J. Anal. At. Spectrom., 2008, 23, 1565–1574, 10.1039/b809755k.
- A. Yamakawa, K. Yamashita, A. Makishima and E. Nakamura, Anal. Chem., 2009, 81, 9787–9794, DOI:10.1021/ac901762a.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ja30083d |
| ‡ Present address: University of Tokyo, Department of Earth and Planetary Sciences, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 Japan. E-mail: E-mail: iizuka@eps.s.u-tokyo.ac.jp; Fax: +81358418378; Tel: +81358414282 |
| This journal is © The Royal Society of Chemistry 2012 |