Cycloisomerisation reactions of γ-alkynoic acids into cyclic enol-lactones can be conveniently performed in pure water as a solvent and under aerobic conditions by using a novel iminophosphorane–Pd(II) complex trans-[PdCl2{μ2-N,S-(PTA)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NP(
NP(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)(OEt)2}]2 as a catalyst. It is important to note that the catalytic system could be recycled up to 10 consecutive runs. In addition and for the first time, a one-pot tandem orthogonal reaction involving the fast cycloisomerisation of γ-alkynoic acids, followed by an intermolecular atom economical process, i.e. the 1,3-dipolar cycloaddition of azides with terminal alkynes (CuAAC), is reported. This new tandem cycloisomerisation/click reaction proceeds also in water, at room temperature and under aerobic conditions, giving rise to unprecedented bicyclic triazol-enol-lactones under the principles of the so called “Green Chemistry”.
S)(OEt)2}]2 as a catalyst. It is important to note that the catalytic system could be recycled up to 10 consecutive runs. In addition and for the first time, a one-pot tandem orthogonal reaction involving the fast cycloisomerisation of γ-alkynoic acids, followed by an intermolecular atom economical process, i.e. the 1,3-dipolar cycloaddition of azides with terminal alkynes (CuAAC), is reported. This new tandem cycloisomerisation/click reaction proceeds also in water, at room temperature and under aerobic conditions, giving rise to unprecedented bicyclic triazol-enol-lactones under the principles of the so called “Green Chemistry”.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NP(
NP(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)(OEt)2}]2 as a
S)(OEt)2}]2 as a 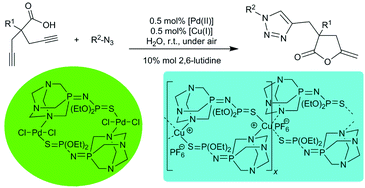

 Please wait while we load your content...
Please wait while we load your content...