Catalytic conversion of biomass using solvents derived from lignin†
Pooya
Azadi
ab,
Ronald
Carrasquillo-Flores
a,
Yomaira J.
Pagán-Torres
a,
Elif I.
Gürbüz
a,
Ramin
Farnood
b and
James A.
Dumesic
*a
aUniversity of Wisconsin-Madison, Department of Chemical and Biological Engineering, Madison, WI 53706, USA. E-mail: dumesic@engr.wisc.edu; Fax: +608-262-5434; Tel: +608-262-1095
bUniversity of Toronto, Department of Chemical Engineering and Applied Chemistry, Toronto, ON M5S 3E5, Canada
First published on 16th March 2012
Abstract
We report an approach by which the hemicellulose and cellulose fractions of biomass are converted through catalytic processes in a solvent prepared from lignin into high value platform chemicals and transportation fuels, namely furfural, 5-hydroxymethylfurfural, levulinic acid and γ-valerolactone.
The production of second-generation biofuels from lignocellulosic biomass can be achieved through the intermediate production of oxygenated platform molecules,1 such as furan intermediates (furfural (FuAl), furfuryl alcohol (FuOH) and 5-hydroxymethylfurfural (HMF)), levulinic acid (LA), and γ-valerolactone (GVL).2 We report herein an approach by which the hemicellulose and cellulose fractions of biomass can be converted to these platform chemicals using an organic solvent obtained by depolymerization of lignin.
Scheme 1 shows our proposed roadmap for the conversion of lignocellulosic biomass to fuels and chemicals. Solid biomass is first subjected to mild pre-treatment in an aqueous solution containing dilute acid to solubilize the hemicellulose as xylose, followed by heating of this aqueous stream to achieve dehydration of xylose to furfural. The use of a biphasic reactor is employed in this step to continuously extract the reactive FuAl from the aqueous phase.3 The remaining biomass is then subjected to further treatment to solubilize cellulose as glucose, and a biphasic reactor is employed in the subsequent step to dehydrate glucose to HMF by enabling the continuous extraction of the reactive HMF product from the acidic aqueous phase.4–7 HMF can then undergo acid hydrolysis to LA and equimolar amounts of formic acid (FA). Similarly, FuAl can be converted to LA by first undergoing reduction to FuOH over a metal catalyst,8,9 followed by conversion to LA over an acid catalyst. The selective hydrolysis of FuOH and HMF to LA is favored at low concentrations of the reactant, and a biphasic reactor can be employed for these conversions, where the function of the organic solvent is to release the reactive reactant continuously into the acidic aqueous phase. In addition, the organic solvent enables extraction of LA from acidic aqueous solutions (e.g., as produced in the Biofine process10), which can then be utilized as a chemical intermediate or can be selectively hydrogenated in the solvent to GVL, the latter serving as a chemical intermediate for polymers and solvents, as well as a blending agent for transportation fuels. The energy density of GVL for use as a transportation fuel can be increased by decarboxylation to form butene, followed by butene oligomerization to gasoline (branched C8 species) or jet fuel (branched C8–C16 species).11
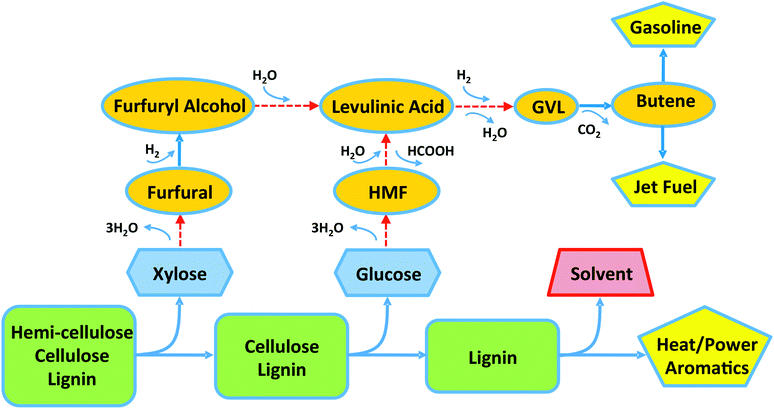 | ||
| Scheme 1 Roadmap for conversion of lignocellulosic biomass (rectangles) to chemicals (ovals) and fuels (pentagons), passing through the intermediate formation of C5 and C6 sugars (hexagons). Dashed arrows indicate processes that can be carried out using a lignin-derived organic solvent for the production of furfural, 5-hydroxymethylfurfural (HMF), levulinic acid, and/or γ-valerolactone (GVL). | ||
An underlying strategy to implement the roadmap outlined in Scheme 1 is to identify organic solvents that can be used to extract biomass-derived products and intermediates (such as FuAl, FuOH, LA, GVL) from acidic aqueous solutions. In our previous work, we have utilized 2-sec-butylphenol (SBP) as an effective solvent.3,12 In the present report, we show that the catalytic depolymerization of lignin can be used to produce a lignin-derived solvent (LDS), containing alkyl-substituted phenolics such as propyl guaiacol (PG) and propyl syringol (PS), that is effective for selective biomass conversion processes, thereby eliminating the need to purchase large amounts of a petroleum-derived solvent and transport it to the biorefinery site. Lignin-derived solvents based on PG and PS have the following attributes to facilitate conversion of the cellulose and hemicellulose fractions of biomass into fuels and chemicals: (i) they have high partition coefficients for extraction of FuAl, FuOH, LA, and GVL from aqueous solutions containing mineral acids, while not extracting significant amounts of mineral acids, and (ii) they have higher boiling points compared to that of the final product (Table S.1†), such that the product is removed at the top of the distillation column used to achieve the final product separation.
We prepared our lignin-derived solvent (LDS) from poplar wood using hot-compressed water under moderate hydrogen pressure (e.g., 4 MPa) utilizing a metal catalyst (i.e., Pd/C, Rh/C and Pt black) to achieve selective hydrogenolysis of C–O–C bonds in a one-pot reactor, followed by extraction from the aqueous phase using diethyl ether (DEE) to obtain a mixture of PG, PS, guaiacyl propanol and syringyl propanol. The final LDS mixture was obtained by evaporating the DEE solvent, followed by the removal of guaiacyl propanol and syringyl propanol from the mixture by extraction with water, leaving a hydrophobic organic phase composed of PS and PG in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio. The total yield of monomers (all four species) obtained from this method is typically 30 wt%, which corresponds to 60% of the theoretical maximum.13 Therefore, with catalytic aqueous phase treatment of 1 kg of dry wood under hydrogen, we can produce 70 g of phenolic monomers. The chemical structures of the major phenolic monomers obtained from the depolymerization of hardwood lignin are depicted in Fig. S.1,† and the selectivity with respect to each monomer over different metal catalysts is given in Table S.2 (see ESI†).
1 mass ratio. The total yield of monomers (all four species) obtained from this method is typically 30 wt%, which corresponds to 60% of the theoretical maximum.13 Therefore, with catalytic aqueous phase treatment of 1 kg of dry wood under hydrogen, we can produce 70 g of phenolic monomers. The chemical structures of the major phenolic monomers obtained from the depolymerization of hardwood lignin are depicted in Fig. S.1,† and the selectivity with respect to each monomer over different metal catalysts is given in Table S.2 (see ESI†).
Table 1 shows results for liquid–liquid extractions of FuAl, FuOH, HMF and LA using PG and LDS in contact with an aqueous phase. For all entries, the extracted amounts in the organic phase are comparable at the two concentrations evaluated. Moreover, the LDS extracts all species with similar effectiveness compared to pure PG. Even though the mass ratio of PG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PS is 1
PS is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 in the LDS, the structural similarity between PG and PS is high, differing only by one methoxy group, thus resulting in similar behavior. The aqueous phase was saturated with NaCl for entries 5, 6, 13 and 14 to increase the partitioning of the target compound into the organic phase.14 Even though the partitioning of LA is low (40%) (entries 7, 8, 15 and 16), the extent of extraction can be improved by using an organic to aqueous mass ratio higher than 1.
4 in the LDS, the structural similarity between PG and PS is high, differing only by one methoxy group, thus resulting in similar behavior. The aqueous phase was saturated with NaCl for entries 5, 6, 13 and 14 to increase the partitioning of the target compound into the organic phase.14 Even though the partitioning of LA is low (40%) (entries 7, 8, 15 and 16), the extent of extraction can be improved by using an organic to aqueous mass ratio higher than 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio with aqueous solutions at 298 K
1 mass ratio with aqueous solutions at 298 K
| Entry | Solvent | Compound | Concentration (M) | % Mole in organic phase | Partition coefficient (Morg/Maq)a |
|---|---|---|---|---|---|
| a Value reported is the average of entries. b The initial aqueous solution was saturated with NaCl to increase the partitioning to the organic phase. c Aqueous solution also contained 0.5 M sulfuric acid. | |||||
| 1 | PG | FuAl | 0.2 | 70 ± 1 | 2.8 ± 0.2 |
| 2 | PG | FuAl | 1.0 | 77 ± 1 | |
| 3 | PG | FuOH | 0.2 | 77 ± 2 | 4.0 ± 0.4 |
| 4 | PG | FuOH | 1.0 | 82 ± 1 | |
| 5 | PG | HMFb | 0.2 | 75 ± 2 | 2.9 ± 0.4 |
| 6 | PG | HMFb | 1.0 | 74 ± 3 | |
| 7 | PG | LAc | 0.7 | 40 ± 1 | 0.68 ± 0.04 |
| 8 | PG | LAc | 2.0 | 41 ± 2 | |
| 9 | LDS | FuAl | 0.2 | 68 ± 3 | 2.6 ± 0.4 |
| 10 | LDS | FuAl | 1.0 | 75 ± 2 | |
| 11 | LDS | FuOH | 0.2 | 78 ± 3 | 3.8 ± 0.6 |
| 12 | LDS | FuOH | 1.0 | 80 ± 2 | |
| 13 | LDS | HMFb | 0.2 | 73 ± 1 | 2.9 ± 0.3 |
| 14 | LDS | HMFb | 1.0 | 75 ± 2 | |
| 15 | LDS | LAc | 0.7 | 39 ± 2 | 0.63 ± 0.04 |
| 16 | LDS | LAc | 2.0 | 38 ± 1 | |
Table 2 shows results for the biphasic dehydration of glucose and xylose to HMF and FuAl, the biphasic hydrolysis of FuOH to LA, and the biphasic hydrolysis of HMF to LA. We have reported elsewhere that SBP in contact with an acidic aqueous phase is an effective biphasic system for reactions of furan compounds.3 The results in Table 2 now show that high selectivities are achieved when PG is used as the extracting solvent. Importantly, high selectivities are also achieved when using LDS prepared from poplar wood.
| Entry | Reactant | wt% | Solvent | O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ab Ab |
T (K) | Conversion (%) | Product | ||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Selectivity (%) | % in organic solvent | |||||||
| a Additional details of the experiments are given in Table S.4 of ESI.† b Organic to aqueous solvent ratio by mass. c Data taken from ref. 3. | |||||||||
| 1 | Glucose | 5 | PG | 2 | 443 | 85 ± 1 | HMF | 58 ± 2 | 82 ± 3 |
| LDS | 82 ± 2 | 59 ± 2 | 88 ± 1 | ||||||
| SBP | 91 ± 2 | 67 ± 2 | 97 ± 1 | ||||||
| 2 | HMF | 1 | PG | 3 | 423 | 96 ± 2 | Levulinic acid | 71 ± 2 | 75 ± 3 |
| LDS | 97 ± 2 | 73 ± 1 | 74 ± 2 | ||||||
| 3 | Xylose | 1.5 | PG | 0.15 | 443 | 75 ± 2 | Furfural | 85 ± 2 | 75 ± 2 |
| LDS | 75 ± 1 | 82 ± 2 | 84 ± 2 | ||||||
| SBPc | 98 ± 2 | 80 ± 2 | 90 ± 2 | ||||||
| 4 | Furfuryl alcohol | 1 | PG | 3 | 398 | 99 ± 1 | Levulinic acid | 63 ± 2 | 73 ± 2 |
| LDS | 99 ± 1 | 65 ± 2 | 76 ± 2 | ||||||
| SBPc | 2 | 100 | 66 ± 4 | 81 ± 5 | |||||
The biphasic dehydration of glucose to HMF (Table 2, entry 1) was carried out starting from 5 wt% glucose in an aqueous 0.1 M HCl solution saturated with NaCl. A Lewis acid (AlCl3 in this case), is utilized to catalyze the isomerization of glucose to fructose,5,15,16 from which HMF is produced in the presence of the mineral acid catalyst. Using PG in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio to the aqueous phase, 58% selectivity towards HMF was obtained at 85% conversion, and 82% of the total HMF is retained in the organic phase. Importantly, the LDS is able to extract up to 88% of HMF, while exhibiting a similar behavior in the selectivity. These results compare well to glucose conversion (91%) and HMF selectivity (67%) obtained using SBP as the extractive organic phase. The partitioning of the majority of the HMF into the organic phase allows for its efficient separation and for the recycling of the mineral acid present in the aqueous phase. Several studies have also demonstrated the effectiveness of biphasic systems in the dehydration of glucose to HMF4,5,17,18 utilizing high boiling organics (for example, dimethyl sulfoxide) with the intention of suppressing side reactions; however, the use of organic extractive solvents containing only C, H, and O atoms, such as the LDS, is advantageous.17
1 mass ratio to the aqueous phase, 58% selectivity towards HMF was obtained at 85% conversion, and 82% of the total HMF is retained in the organic phase. Importantly, the LDS is able to extract up to 88% of HMF, while exhibiting a similar behavior in the selectivity. These results compare well to glucose conversion (91%) and HMF selectivity (67%) obtained using SBP as the extractive organic phase. The partitioning of the majority of the HMF into the organic phase allows for its efficient separation and for the recycling of the mineral acid present in the aqueous phase. Several studies have also demonstrated the effectiveness of biphasic systems in the dehydration of glucose to HMF4,5,17,18 utilizing high boiling organics (for example, dimethyl sulfoxide) with the intention of suppressing side reactions; however, the use of organic extractive solvents containing only C, H, and O atoms, such as the LDS, is advantageous.17
The hydrolysis of HMF to LA has previously been studied in a single-phase system where HCl and H2SO4 have been identified as the best catalysts for the system resulting in high yields (i.e., 94%).19 However, purification of LA and recovery of the mineral acid is an energy intensive process involving solvent extraction combined with distillation steps. The application of our biphasic processing strategy using PG and LDS, for an organic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous mass ratio of 3
aqueous mass ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 2, entry 2), results in high selectivities (72%) for production of LA at almost complete conversion, allowing the processing of HMF in the extracting solvent without the need of a purification step. Additionally, the majority of the produced LA (75%) is recovered in the organic phase, enabling the separation of LA from the mineral acid in the aqueous phase.
1 (Table 2, entry 2), results in high selectivities (72%) for production of LA at almost complete conversion, allowing the processing of HMF in the extracting solvent without the need of a purification step. Additionally, the majority of the produced LA (75%) is recovered in the organic phase, enabling the separation of LA from the mineral acid in the aqueous phase.
Analogous to the dehydration of glucose, conversion of xylose to FuAl has been previously studied in biphasic systems.7,20 These systems utilized organic solvents (e.g., methyl isobutyl ketone, 2-butanol and tetrahydrofuran), which suffer from low partitioning of FuAl into the organic phase. Using alkylphenol solvents (e.g., SBP) that have high partition coefficients for FuAl, it is possible to concentrate the FuAl to several times the initial concentration of xylose in the aqueous phase by using small amounts of the extractive solvent relative to the aqueous layer.3 We demonstrate here that both PG and the LDS perform in a manner that is similar to the SBP solvent (Table 2, entry 3), resulting in 85% and 82% selectivities to FuAl, respectively, at 75% xylose conversion, using an organic to aqueous mass ratio equal to 0.15. The partitioning of FuAl into the organic layer leads to 75% and 84% of the FuAl being retained in PG and LDS, respectively.
The FuAl in the organic phase can be distilled out at this point to serve as an end product or it can undergo subsequent hydrogenation in the gas phase over a metal catalyst to form FuOH,8,9 which can be converted in another biphasic reactor to LA. The yield of LA by hydrolysis of FuOH is limited by polymerization reactions, even at low concentrations. Thus, we implemented a biphasic reactor system where the FuOH is slowly partitioned into the aqueous phase from the organic phase. Due to the high partitioning of FuOH into PG and the LDS, it is possible to minimize the concentration of FuOH in the aqueous phase. The LA yields obtained at complete conversion using PG (63% yield, with 73% of LA being in PG) and the LDS (65% yield, with 76% of LA being in LDS) compare well with experiments using SBP as a solvent, where 81% of the LA was partitioned into the organic medium with comparable yields (∼65%).3 As shown previously by Lange et al.21 and Gürbüz et al.,3 a semi-batch operation mode can be adopted to process higher concentrations (∼10–20 wt%) of furfuryl alcohol.
Scheme 1 shows the hydrogenation of LA to GVL as a step where both the C5 and C6 pathways can be combined and processed into transportation fuels. Several publications have addressed this reaction with a variety of methods.12,22,23 In the present study, we studied the conversion of LA to GVL, using an organic stream containing LA and FA in PG that was prepared by contacting PG with an aqueous stream of LA and FA prepared to match the effluent composition from the acid-catalyzed hydrolysis of cellulose. Table 3 shows the effects of temperature and weight hourly space velocity (WHSV) on the hydrogenation of LA to GVL in PG solvent using a Ru/C catalyst in a packed bed flow reactor. Increasing the temperature from 453 to 473 K increased the conversion of LA as well as the conversion of PG, from 62% to 82% and from 0.5% to 3.6%, respectively. In a similar manner, lowering the WHSV of the feed from 2.2 to 1.2 h−1 affected the extent of hydrogenation for both LA and PG, with an increase from 62% to 100% and from 0.5% to 6.0%, respectively. Hydrogenation of PG solvent could be completely suppressed with the addition of Sn to 5 wt% Ru/C in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 Ru
4 Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn molar ratio as demonstrated previously for SBP.12,24 Even though the addition of Sn decreased the GVL production rate (3.4 mmol g−1 h−1), RuSn4/C exhibited good stability for 80 h of operation at around 96% LA conversion, as shown in Fig. 1.
Sn molar ratio as demonstrated previously for SBP.12,24 Even though the addition of Sn decreased the GVL production rate (3.4 mmol g−1 h−1), RuSn4/C exhibited good stability for 80 h of operation at around 96% LA conversion, as shown in Fig. 1.
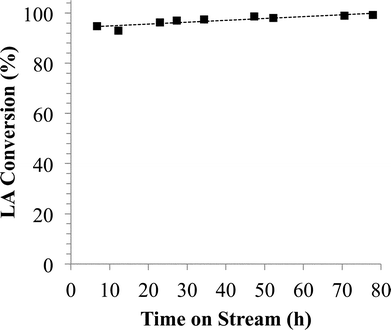 | ||
| Fig. 1 Levulinic acid conversion versus time on stream over RuSn4/C at 453 K, 34.5 bar H2 pressure and WHSV= 0.4 h−1. Feed is obtained using PG to extract LA and FA from aqueous solution, according to entry 2 in Table S.3.† | ||
| Entry | T (K) | WHSVb (h−1) | Conversion (%) | GVL selectivity (%) | GVL rate (mmol/gcat h−1) | |
|---|---|---|---|---|---|---|
| LA | PG | |||||
| a Feed composition corresponds to entry 2 in Table S.3:† LA/FA = 42%/9%. H2 pressure in the reactor was fixed at 13.8 bars. b WHSV is defined as mass of LA fed per mass of catalyst per hour. | ||||||
| 1 | 453 | 2.2 | 62 | 0.5 | 96 | 13.6 |
| 2 | 473 | 2.1 | 82 | 3.6 | 94 | 17.2 |
| 3 | 453 | 1.2 | 100 | 6.0 | 93 | 13.2 |
In summary, the use of lignin-derived alkylphenols as solvents in biphasic reactors enables in situ separation of products and/or reactants, thereby minimizing undesirable side reactions in the aqueous phase and enabling the recycling of the mineral acid catalysts in biomass processing. The overall processing strategy presented here could benefit from further optimization of lignin depolymerization processes to increase the yields obtained in the production of alkylphenol compounds. In addition, while the Ru/C catalyst is effective for the conversion of LA to GVL in the lignin-derived solvent, it is possible to use bimetallic catalysts, such as RuSn, to further minimize the rates at which the solvent is hydrogenated during the conversion of LA to GVL and to ensure stable activity.
Acknowledgements
This material is based upon work supported as part of the Institute for Atom-efficient Chemical Transformations (IACT), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, and work supported by the DOE Great Lakes Bioenergy Research Center (http://www.greatlakesbioenergy.org), which is supported by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, through Cooperative Agreement DE-FC02-07ER64494 between The Board of Regents of the University of Wisconsin System and the U.S. Department of Energy. We also received funding from the National Science Foundation under Award No. EEC-0813570 (i.e., the Center for Biorenewable Chemicals, CBiRC, at Iowa State University). The authors thank Zuleika Oquendo for her assistance in the performed investigations. Also, P. A. acknowledges financial support from the NSERC Michael Smith scholarship.Notes and references
- D. Alonso, J. Bond and J. Dumesic, Green Chem., 2010, 12, 1493–1513 RSC.
- I. Horvath, H. Mehdi, V. Fabos, L. Boda and L. Mika, Green Chem., 2008, 10, 238–242 RSC.
- E. I. Gürbüz, S. G. Wettstein and J. A. Dumesic, ChemSusChem, 2012, 5, 383–387 CrossRef.
- Y. Roman-Leshkov, J. Chheda and J. Dumesic, Science, 2006, 312, 1933–1937 CrossRef CAS.
- E. Nikolla, Y. Roman-Leshkov, M. Moliner and M. Davis, ACS Catal., 2011, 1, 408–410 CrossRef CAS.
- J. Chheda and J. Dumesic, Catal. Today, 2007, 123, 59–70 CrossRef CAS.
- J. Chheda, Y. Roman-Leshkov and J. Dumesic, Green Chem., 2007, 9, 342–350 RSC.
- B. Nagaraja, A. Padmasri, B. Raju and K. Rao, J. Mol. Catal. A: Chem., 2007, 265, 90–97 CrossRef CAS.
- R. Rao, R. Baker and M. Vannice, Catal. Lett., 1999, 60, 51–57 CrossRef CAS.
- S. W. Fitzpatrick, US Pat., 5608105, 1997 Search PubMed.
- J. Q. Bond, D. Martin-Alonso, D. Wang, R. M. West and J. A. Dumesic, Science, 2010, 327, 1110–1114 CrossRef CAS.
- D. Alonso, S. Wettstein, J. Bond, T. Root and J. Dumesic, ChemSusChem, 2011, 4, 1078–1081 CrossRef CAS.
- N. Yan, C. Zhao, P. J. Dyson, C. Wang, L. T. Liu and Y. Kou, ChemSusChem, 2008, 1, 626–629 CrossRef CAS.
- Y. Roman-Leshkov and J. Dumesic, Top. Catal., 2009, 52, 297–303 CrossRef CAS.
- H. Zhao, J. Holladay, H. Brown and Z. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS.
- M. Chidambaram and A. Bell, Green Chem., 2010, 12, 1253–1262 RSC.
- Y. Roman-Leshkov, C. Barrett, Z. Liu and J. Dumesic, Nature, 2007, 447, 982–985 CrossRef CAS.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS.
- B. Girisuta, L. Janssen and H. Heeres, Green Chem., 2006, 8, 701–709 RSC.
- R. Xing, A. Subrahmanyam, H. Olcay, W. Qi, G. van Walsum, H. Pendse and G. Huber, Green Chem., 2010, 12, 1933–1946 RSC.
- J. Lange, W. van de Graaf and R. Haan, ChemSusChem, 2009, 2, 437–441 CrossRef CAS.
- J. Serrano-Ruiz, D. Wang and J. Dumesic, Green Chem., 2010, 12, 574–577 RSC.
- J. Serrano-Ruiz, D. Braden, R. West and J. Dumesic, Appl. Catal., B, 2010, 100, 184–189 CrossRef CAS.
- S. G. Wettstein, J. Q. Bond, D. M. Alonso, H. N. Pham, A. K. Datye and J. A. Dumesic, Appl. Catal., B, 2012, 117, 321–329 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Lignin depolymerization, liquid–liquid extractions, biphasic reactions, selective hydrogenation of LA to GVL. See DOI: 10.1039/c2gc35203f |
| This journal is © The Royal Society of Chemistry 2012 |
