Efficient bio-conversion of glycerol to glycerol carbonate catalyzed by lipase extracted from Aspergillus niger
Madalina
Tudorache
,
Loredana
Protesescu
,
Simona
Coman
and
Vasile I.
Parvulescu
*
University of Bucharest, Department of Organic Chemistry, Biochemistry and Catalysis, Bd. Regina Elisabeta 4–12, Bucharest, 030016, Romania. E-mail: vasile.parvulescu@g.unibuc.ro; Fax: 4021 4100241; Tel: 4021 4100241
First published on 5th January 2012
Abstract
A biocatalytic synthesis of glycerol carbonate (GlyC), as an added-value product of renewable glycerol, has been developed using a catalytic route in which glycerol (Gly) was reacting with dimethyl carbonate (DMC) in the presence of lipase under solvent-free conditions. The enzyme screening indicated lipase from Aspergillus niger as the most efficient biocatalyst for the GlyC synthesis. After the optimization of the reaction conditions it was established that the best results corresponded to 12% (w/w) Aspergillus nigerlipase, to a glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC molar ratio of 1
DMC molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, to an incubation time of 4 h and to an incubation temperature of 60 °C. Consequently, the glycerol conversion was around 74%, the yield in GlyC of 59.3% and the selectivity to GlyC of 80.3%. Recycling experiments demonstrated that the biocatalyst can be successfully used for several reaction cycles (at least 4 times) and confirmed its very high stability under the reaction conditions.
10, to an incubation time of 4 h and to an incubation temperature of 60 °C. Consequently, the glycerol conversion was around 74%, the yield in GlyC of 59.3% and the selectivity to GlyC of 80.3%. Recycling experiments demonstrated that the biocatalyst can be successfully used for several reaction cycles (at least 4 times) and confirmed its very high stability under the reaction conditions.
Introduction
The current requests of the chemical industry assume the development of green synthetic routes involving renewable raw materials and the replacement of “unfriendly” syntheses, which usually generate toxic residues and/or involve harsh reaction conditions, with friendly ones. Nowadays glycerol is generated as a by-product at low prices (e.g. from the production of biodiesel using biomass as a raw material). However, it is considered as one of the main candidates to be used as a building block in chemical synthesis. Thus, it is already considered as a platform molecule for fine syntheses, and the possibility to transform it into a large number of high-value chemicals, such as classic esters and oligomers, and also into new products like telomers, branched alkyl ethers, propanediols, epoxides and glycerol carbonate has been proved.1,2Glycerol carbonate (4-hydroxymethyl-1,3-dioxolan-2-one, GlyC) is a relatively new compound in the chemical industry with great potential in a large synthesis area. Thus GlyC is considered a new “green” solvent due to its ideal physico-chemical properties, such as high stability, low toxicity, low evaporation rate and low flammability3 and the possibility to use it as a solvent in cosmetics, personal care items, and medicine has been suggested.4GlyC is also a valuable intermediate for the production of resins and plastics, and in the pharmaceutical and cosmetics industry after its transformation into glycidol.5GlyC represents the main electrolyte ingredient of lithium-based batteries6 and can be used for the production of coatings, adhesives and lubricants viapolymerization or via reaction with izocyanates/acrylates.7
Nowadays, the industrial production of GlyC takes place following two reaction steps involving the synthesis of a cyclic ethylene carbonate followed by its reaction with glycerol.1
However, these procedures present several economic and environmental drawbacks that require improvements to achieve the desired feasibility. A possible alternative for the green production of GlyC is glycerolation of dimethyl carbonate (DMC) with glycerol (Gly) using either inorganic or bio-catalysts (Scheme 1). Glycerol has two types of OH groups exhibiting different reactivity: the two primary alcohol groups are presumably more reactive than the secondary OH group. Accordingly, the reaction mechanism of glycerolation is supposed to follow the route described in Scheme 1. Glycerol carboxylation leads to an unstable intermediate (3), which forms the GlyC (4) product through an intramolecular conversion. The reaction can continue to glycerol dicarbonate (GlyDC) (5) and furthermore to diglycerol tricarbonate (DGlyTC) (6) when the excess of DMC is substantially high.7
 | ||
| Scheme 1 Glycerol carbonate (GlyC) synthesis based on DMC glycerolation with glycerol (Gly). | ||
Both reagents (e.g. Gly and DMC) are non-toxics, biodegradables and are generated through clean production processes. Chemo-catalytic synthesis of GlyC based on the reaction of Gly with DMC has been investigated in both homogeneous conditions using alkali-based catalysts (e.g. potassium carbonate)7 and heterogeneous conditions using solid calcium materials or precursors of these (e.g.calcium oxide, calcium hydroxide or calcium methoxide).8,9 Recently, for the synthesis of GlyC, the use of lipases (extracted from Candida antarctica) was reported as well,10,11 thus moving the reaction conditions to the mild ones and the selectivity to high values. One of these reports proposed the transesterification of glycerol with DMC in tetrahydrofuran (THF).11 The second one indicated the synthesis of GlyC under solvent-free conditions with DMC playing a double role: substrate and solvent.10
In this study, we extended the scope of the synthesis of GlyC under solvent-free conditions. We report a screening of the catalytic performances of various lipases extracted from Aspergillus niger, Pseudomonas fluorescens, Phizopus arrhizus, Candida cylindracea, Pseudomonas cepacia, Mucor miehei, Aspergillus sp, Porcina pancreas, Phizopus niveus, Hog pancreas and Thermomyces lonuginosus. The experiments have been carried out considering as reference a lipase extracted from Candida antarctica. After the selection of the most performant enzyme (Aspergillus nigerlipase), the second objective was to optimize the synthesis conditions and to check the recyclability, and implicitly, the stability of the bio-catalyst.
Results and discussion
Screening of biocatalyst for glycerol carbonate synthesis
The catalytic activity of lipase from various natural sources has been directly evaluated in the transesterification of glycerol with DMC under solvent-free conditions. Using this strategy, the influence of DMC, as solvent, on the lipase activity has also been evaluated. The collected data showed that among the tested lipases, Pseudomonas cepacia, Candida antarctica and Aspergillus niger were the only sources expressing lipase enzyme able to conserve at a high level the catalytic activity in DMC solvent (Fig. 1). These data confirm results already reported showing Candida antarctica as an efficient lipase source for the GlyC synthesis.10–12 As it was mentioned above this was used as reference in our study. However, the screening of the lipases presented in Fig. 1 showed that Aspergillus niger represents a more efficient lipase source then Pseudomonas cepacia and Candida antarctica. Therefore, Aspergillus niger was selected for the optimization of the GlyC synthesis. All other sources led to poorer results (Fig. 1). | ||
| Fig. 1 Screening the lipase sources for glycerol carbonate synthesis. Reaction conditions: 0.1 g glycerol, 1 mL DMC and 0.02 g enzyme; temperature 60 °C; 48 h incubation time. | ||
Optimization of the reaction conditions
Fig. 2 shows results for the synthesis of GlyC using different molar ratios of Gly to DMC. Theoretically, the reaction stoichiometry requires an equimolar ratio of reagents (i.e. of Gly-to-DMC, see Scheme 1). However, the mechanism of the reaction is strongly influenced by the catalyst type (e.g. inorganic structure or enzyme) and the reaction conditions. Base inorganic catalysts (e.g.K2CO3) favor the formation of GlyC using only a 3 fold excess of DMC. Secondary products (e.g. GlyDC and DGlyTC) resulted for a higher excess of DMC (10 fold) and at high temperatures (e.g. 95 °C).7 Using enzymes, the optimum molar ratio of Gly to DMC that has been reported was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 when the reaction was carried out in THF solvent, and a much smaller (1
2 when the reaction was carried out in THF solvent, and a much smaller (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) for solvent-free systems.10,11
10) for solvent-free systems.10,11
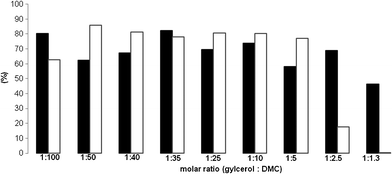 | ||
| Fig. 2 Influence of reagents molar ratio on the synthesis of GlyC (■ – glycerol conversion (%), □ – GlyC selectivity (%)). Conditions: 0.1 g glycerol; 12% (w/w) lipase; 60 °C temperature; 48 h incubation time. | ||
The synthesis of GlyC in the presence of Aspergillus nigerlipase followed the same tendency. Thus, for an equimolar ratio of Gly to DMC, the Gly conversion was of only 46.4% without the formation of the GlyC product (Fig. 2). In this case, we suppose that only one glycerol “–OH” group reacts with DMC. Consequently, the DMC could couple two glycerol molecules due to the glycerol excess. This supposition is in agreement with the mechanism of DMC glycerolation proposed by Rokicki et al.7 The decrease of the Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC ratio led to both an increase of the conversion and to the formation of the desired product with an increased selectivity. Indeed, it was found that a molar ratio of 1
DMC ratio led to both an increase of the conversion and to the formation of the desired product with an increased selectivity. Indeed, it was found that a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 corresponds to an optimum under these conditions. For Aspergillus niger it corresponded to a Gly conversion of 73.8% and a selectivity to GlyC of 80%. Further decrease of this ratio till a Gly
10 corresponds to an optimum under these conditions. For Aspergillus niger it corresponded to a Gly conversion of 73.8% and a selectivity to GlyC of 80%. Further decrease of this ratio till a Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC of 1
DMC of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 led to only a very small increase of the Gly conversion (80%) but to a significant depreciation of the GlyC selectivity (62%).
100 led to only a very small increase of the Gly conversion (80%) but to a significant depreciation of the GlyC selectivity (62%).
Fig. 3 illustrates the effect of the enzyme concentration in this reaction. The plot profile indicates a gradual increase of the process efficiency (Gly conversion and yield in GlyC) when the enzyme concentration raise from 5 to 12% (w/w) (Gly conversion of 81.3% and yield in GlyC of 60.4% for 12% lipase concentration (w/w)). The selectivity suffers a slight decrease in this range of catalyst concentrations (around 25%). A further increase of the enzyme loading till 15% (w/w) had no effect on Gly conversion and selectivity to GlyC, but the yield to GlyC decreased with around 5%. 12% (w/w) seems to be not only an economic limit of the catalyst loading, but also in terms of performances. Thus, higher concentrations than 15% (w/w) led to a decrease of the monitored parameters (conversion, selectivity and yield). It was thus found that a high enzyme density causes inter-molecular interactions of lipase, in this way blocking the catalytic sites. Comparing the data with Aspergillus nigerlipase obtained in this study with those already reported in the literature with the lipase from Candida Antarctica (either in THF (21% (w/w)11 or under free-solvent conditions (33% (w/w)10) it appears that the lipase from Aspergillus niger is, indeed, a more efficient catalyst.
 | ||
Fig. 3
Lipase concentration in the reaction mixture (♦ – glycerol conversion (%), ● – GlyC yield (%), ■ – GlyC selectivity (%)). Conditions: 0.1 g glycerol; glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC molar ratio = 1 DMC molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; temperature 60 °C; 48 h incubation time. 10; temperature 60 °C; 48 h incubation time. | ||
Effect of incubation time and temperature on GlyC synthesis
Fig. 4 shows the variation of the glycerol conversion, yield in GlyC and selectivity to GlyC after different incubation times. The reaction almost establishes the equilibrium after 4 h (74% Gly conversion, 58% GlyC yield and 80% GlyC selectivity). Further increase of the incubation time to 48 h led to an increase of the conversion with 10%, while the yield remained nearly the same (Fig. 4). However, the selectivity is a key factor of the process and the increase of the incubation time to 48 h generated a continuous decrease due to the formation of secondary products. It appears thus that an incubation time of 4 h provides the best compromise between the conversion of Gly and the selectivity to GlyC. It is also worth to mention that using Candida antarcticalipase a conversion of glycerol of 74% was achieved only after 25 h.10 | ||
| Fig. 4 Influence of the incubation time on the GlyC synthesis (■ – glycerol conversion (%), – GlyC yield (%), □ – GlyC selectivity (%)). Conditions: 0.1 g glycerol; glycerol ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC molar ratio = 1 DMC molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; 12% (w/w) lipase; temperature – 60 °C. 10; 12% (w/w) lipase; temperature – 60 °C. | ||
The influence of the temperature on the efficiency of the GlyC synthesis has also been investigated. The experimental results are summarized in Fig. 5. Both the conversion of Gly and the yield to GlyC increased gradually with the temperature till a maximum at 60 °C (73.8% conversion of Gly and 59.3% yield to GlyC). Higher temperatures than 60 °C led to a drastic decrease of the reaction performances, most likely, due to the denaturation of the enzyme structure.13 Also, the selectivity to GlyC showed a slow decrease when the temperature varied between 25 and 60 °C (from 95% to 80%, respectively). However, the selectivity of the process presented a sudden drop for temperatures higher than 60 °C, which also corresponds to an enzyme inhibition by structure denaturation.
 | ||
| Fig. 5 Temperature effect on the evolution of the GlyC synthesis process ( – glycerol conversion, □ – yield in GlyC, ■ – selectivity to GlyC). Conditions: 0.1 g glycerol; glycerol ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC molar ratio = 1 DMC molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; 12% (w/w) lipase; temperature 60 °C; 48 h incubation time. 10; 12% (w/w) lipase; temperature 60 °C; 48 h incubation time. | ||
The TOF (turnover frequency) of the lipase from Aspergillus niger under optimum conditions of the GlyC synthesis was of 1.16 × 105 h−1.
Biocatalyst stability and recyclability
study involves a two phase configuration with DMCThe synthesis of GlyC using the methodology reported in this study involves a two phase configuration with DMC corresponding to the hydrophobic phase and Gly to the hydrophilic phase. The biocatalyst (Aspergillus nigerlipase) was dispersed in the reaction volume. At the end of the reaction, the enzyme was easily recovered from the reaction mixture by centrifugation and the catalyst was recycled in another GlyC synthesis. The operational stability of Aspergillus nigerlipase was thus investigated in five successive reaction cycles (Table 1). The experimental data showed no significant changes in the biocatalyst activity after four cycles. Till the fourth cycle, the loss of the conversion was of maximum 4% while the yield in GlyC decreased with less than 5%. However, after the fourth cycle the enzyme rapidly lost the activity. Both the Gly conversion and the yield in GlyC were diminished under these conditions to half of the initial values (Table 1). The behavior is reproducible and requires more characterization evidence to explain the abrupt behavior.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC molar ratio = 1
DMC molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; 12% (w/w) lipase; temperature 60 °C; 1 h incubation time
10; 12% (w/w) lipase; temperature 60 °C; 1 h incubation time
Conclusions
A solvent-free synthesis of GlyC has been developed based on glycerolation of DMC catalyzed by a lipase enzyme from an Aspergillus niger source. The main advantages of the developed synthesis method are (1) the strategy follows an eco-friendly and non-toxic route (i.e. the reaction mixture before and after synthesis does not contain pollutants); (2) it corresponds to an efficient synthesis since a low enzyme concentration is required (only 12% (w/w) lipase) and the enzyme stability and recyclability under operation conditions have been proved for four reaction cycles; the chemical reaction was carried out under mild conditions (60 °C and atmospheric pressure), with a convenient atomic economy; (3) it leads to a performant synthesis process for GlyC production (conversion of glycerol of 73.8%, yield to GlyC of 59.3% and selectivity to GlyC of 80.3%).Experimental
Chemicals and enzymes
The substances used in this study: glycerol (Gly) and dimethyl carbonate (DMC) were purchased from Sigma-Aldrich (USA). Lipase enzymes from different sources (e.g. Aspergillus niger, Candida antarctica, Pseudomonas fluorescens, Phizopus arrhizus, Candida cylindracea, Pseudomonas cepacia, Mucor miehei, Aspergillus sp, Porcina pancreas, Phizopus niveus, Hog pancreas and Thermomyces lonuginosus) were also obtained from Sigma-Aldrich (USA). Derivatization reagents (BSTFA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMCS = 99
TMCS = 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and pyridine) were purchased from Macherey-Nagel Corp. (Duren, Germany) and Fluka (Switzerland). The organic solvents used in all the experiments were of analytical purity.
1 and pyridine) were purchased from Macherey-Nagel Corp. (Duren, Germany) and Fluka (Switzerland). The organic solvents used in all the experiments were of analytical purity.
Bio-catalytic synthesis of glycerol carbonate (GlyC)
Enzymatic synthesis of GlyC has been performed in a solvent-free system. Given amounts of Gly and DMC at different Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC molar ratios (1
DMC molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 1
25, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and 1
50 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) were mixed together with the lipase catalyst (2.58, 5.04, 7.38, 11.72, 15.15, 21.13, 26.32%, w/w) in a 1.5 mL reaction vial (Eppendorf tube). The mixtures were incubated for a maximum of 48 h under stirring at temperatures in the range 30–80 °C using a thermostatted shaker. After the reaction was completed, the suspension was centrifuged to recover the enzyme. The filtrated liquid phase was evaporated at 50 °C under vacuum for the elimination of the DMC excess and methanol. Finally, only dried reaction products (e.g.GlyC and secondary products) and unreacted Gly were found in the vial. The recovered enzyme was used for the next round of the reaction.
100) were mixed together with the lipase catalyst (2.58, 5.04, 7.38, 11.72, 15.15, 21.13, 26.32%, w/w) in a 1.5 mL reaction vial (Eppendorf tube). The mixtures were incubated for a maximum of 48 h under stirring at temperatures in the range 30–80 °C using a thermostatted shaker. After the reaction was completed, the suspension was centrifuged to recover the enzyme. The filtrated liquid phase was evaporated at 50 °C under vacuum for the elimination of the DMC excess and methanol. Finally, only dried reaction products (e.g.GlyC and secondary products) and unreacted Gly were found in the vial. The recovered enzyme was used for the next round of the reaction.
Analyses
The reaction products were analyzed using gas chromatography (GC) coupled to mass-spectrometry (MS) or flame ionization (FID) detectors. While GC-MS allowed the identification of the reaction products, GC-FID led to a quantitative evaluation of the reaction mixture composition at the end of the synthesis.The analysis of glycerol and reaction products required silylation before the injection on the chromatographic column, in order to obtain proper peak shapes, and also a low detection limit.14 For this purpose, 100 μL silylation agent (BSTFA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMCS = 99
TMCS = 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were added to the reaction samples (after the evaporation step), and then the resulted mixture was diluted with 100 μL pyridine. The derivatization process has been performed under gently agitation, at 60 °C, for 30 min. Before analysis, 100 μL of n-heptane was added as internal standard.
1) were added to the reaction samples (after the evaporation step), and then the resulted mixture was diluted with 100 μL pyridine. The derivatization process has been performed under gently agitation, at 60 °C, for 30 min. Before analysis, 100 μL of n-heptane was added as internal standard.
Derivatized samples (1 μL) were analyzed with a GC-FID (Schimadzu GC-2014, Thermo Electron Scientific Corporation, USA) chromatograph equipped with TR-WAX and TR1MS capillary columns using hydrogen as a carrier gas (1.0 mL min−1). The detector and injector were set up at the temperature of 250 °C. The temperature in the oven was kept constant at 50 °C for 1 min and then was increased to 250 °C with a 10 °C min−1 rate. Finally, the oven temperature was maintained at 250 °C for 3 min. GC-MS analyses were performed with a Trace GC 2000 system with MS detector (Thermo Electron Scientific Corporation, USA) incorporating a TR-WAX capillary column. The injection chamber was set up at 200 °C and the temperature in the detector cell was 270 °C. The oven program was similar with that used for the GC-FID analysis. The GC-MS chromatogram and the corresponding spectra of silylated samples were used to identify the reaction products. MS (m/z) (1) silylated glycerol carbonate: 190 (M+), 145 (M+ − 3CH3), 131 (M+ − Si(CH3)2), 117 (M+ − Si(CH3)3), 101 (M+ − OSi(CH3)3+), 89 (OSi(CH3)3+), 73 (Si(CH3)3+).9
The conversion of Gly (CGly), the yield in GlyC and the selectivity to GlyC were calculated using equations (1–3), where the number of moles was determined from the chromatographic analysis.
| CGly(%) = (sum of the moles of reaction products)/(moles of Gly introduced in the reaction) × 100 | (1) |
| GlyC yield(%) = (moles of GlyC/moles of Gly introduced in the reaction) × 100 | (2) |
| GlyC selectivity (%) = (moles of GlyC/sum of the moles of reaction products) × 100 | (3) |
The characterization of the reaction products was also performed by FTIR spectrometry using a Thermo 4700 spectrometer (Thermo Electron Scientific Corporation, USA). The samples were analyzed after the DMC excess has been removed (dried samples prepared under vacuum conditions). The spectra of the samples containing GlyC exhibited a band at 1789 cm−1 corresponding to carbonyl stretching mode of glycerol carbonate.9,15
Acknowledgements
This work was financially supported by PN II HR YT-96 program, contract no. 91/2010 from CNCSIS.References
- A. Behr, J. Eilting, K. Irawadi, J. Leschinski and F. Lindner, Green Chem., 2008, 10, 13–30 RSC.
- M. Pagliaro, R. Ciriminna, H. Kimura, M. Rossi and C. Della Pina, Angew. Chem., Int. Ed., 2007, 46, 4434–4440 CrossRef CAS.
- J. Huntsman Corporation, Glycerine Carbonate, 2009, http://www.huntsman.com/performanceproducts/Media/JEFFSOL%C2%AEGlycerineCarbonate.pdf.
- D. Herault, A. Eggers, A. Strube and J. Reinhard, Ger. Offen., 2002, DE 10110855 Search PubMed.
- J. Rousseau, C. Rousseau, B. Lynikaite, A. Sakcus, C. de Leon, P. Rollin and A. Tatibouet, Tetrahedron, 2009, 65, 8571–8581 CrossRef CAS.
- A. S. Kovvali and K. K. Sirkar, Ind. Eng. Chem. Res., 2002, 41, 2287–2295 CrossRef CAS.
- G. Rokicki, P. Rakoczy, P. Parzuchowski and M. Sobiecki, Green Chem., 2005, 7, 529–539 RSC.
- J. Li and T. Wang, React. Kinet., Mech. Catal., 2011, 102, 113–126 CrossRef CAS.
- J. R. Ochoa-Gómez, C. Ramírez-López, M. C. Villarán-Velasco, L. Lorenzo-Ibarreta, A. Pesquera-Rodríguez, O. Gómez-Jiménez-Aberasturi, J. Torrecilla-Soria and B. Maestro-Madurga, Appl. Catal., A, 2009, 366, 315–324 CrossRef.
- E. Y. Lee, K. H. Lee and C.-H. Park, Bioprocess Biosyst. Eng., 2010, 33, 1059–1065 CrossRef.
- D. Y. Yoon, S. C. Kim, H. Lee, B. K. Song and Y. H. Kim, J. Mol. Catal. B: Enzym., 2007, 49, 75–78 CrossRef.
- D Royon, M Daz, G Ellenrieder and S. Locatelli, Bioresour. Technol., 2007, 98, 648–653 CrossRef CAS.
- R. Sharmaa, Y. Chistib and U. Chand Banerjee, Biotechnol. Adv., 2001, 19, 627–662 CrossRef.
- C. Plank and E. Lorbeer, J. Chromatogr., A, 1995, 697, 461–468 CrossRef CAS.
- M. Aresta, A. Dibenedetto, F. Nocito and C. Ferragina, J. Catal., 2009, 268, 106–114 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |


