Evaluation of metallothionein formation as a proxy for zinc absorption in an in vitro digestion/Caco-2 cell culture model
Zhiqiang
Cheng
a,
Elad
Tako
*a,
Andrew
Yeung†
b,
Ross M.
Welch
a and
Raymond P.
Glahn
a
aUSDA- ARS Robert W. Holley Center for Agricultural and Health, Cornell University, Ithaca, NY, USA 14853. E-mail: et79@cornell.edu; Fax: +1-607-255-1132; Tel: +1-607-339-6542
bDept. of Food Science, Cornell University, Ithaca, NY, USA 14853
First published on 27th June 2012
Abstract
Caco-2 cell metallothionein (MT) formation was studied to determine if MT could be used as a proxy for zinc (Zn) absorption in a cell culture model. The MT intracellular concentration was determined using a cadmium/hemoglobin affinity assay. The cellular Zn uptake was determined by acid digests (5% HNO3) using inductively-coupled argon-plasma emission spectroscopy. The effect of phytic acid (PA) on cellular Zn and MT concentrations was also studied. Cells were treated with a media containing 0, 2, 5, 10, 25, 50, 75 μmol L−1 Zn (ZnCl2). The effect of varying the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA molar ratios (1
PA molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1
0, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) on the Zn uptake and MT formation was determined. The results showed a positive linear correlation between Zn-media concentrations and cellular Zn uptake, and MT formation was observed. Zn and MT concentrations in the cells treated with increasing levels of Zn (>25 μmol L−1 Zn) were elevated. The Zn and MT concentrations in the cells incubated with Zn (when <10 μmol L−1) were similar to the untreated cells. PA significantly lowered the cellular Zn and MT concentrations. When the Zn
20) on the Zn uptake and MT formation was determined. The results showed a positive linear correlation between Zn-media concentrations and cellular Zn uptake, and MT formation was observed. Zn and MT concentrations in the cells treated with increasing levels of Zn (>25 μmol L−1 Zn) were elevated. The Zn and MT concentrations in the cells incubated with Zn (when <10 μmol L−1) were similar to the untreated cells. PA significantly lowered the cellular Zn and MT concentrations. When the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA molar ratios were >1
PA molar ratios were >1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, cellular MT concentrations were no different to untreated cells. When a combined in vitro digestion/cell model was used, the cellular MT concentrations in white or red beans and fish samples were no different to the cell baseline. This study suggests that measurements of cellular Zn and MT concentrations have some limitations (<10 μmol L−1 Zn). PA was observed to be a potent inhibitor of Zn uptake. Under the conditions of this in vitro model, Caco-2 cell monolayers are not useful for evaluating the Zn availability from foods.
5, cellular MT concentrations were no different to untreated cells. When a combined in vitro digestion/cell model was used, the cellular MT concentrations in white or red beans and fish samples were no different to the cell baseline. This study suggests that measurements of cellular Zn and MT concentrations have some limitations (<10 μmol L−1 Zn). PA was observed to be a potent inhibitor of Zn uptake. Under the conditions of this in vitro model, Caco-2 cell monolayers are not useful for evaluating the Zn availability from foods.
1. Introduction
Zinc (Zn) is an essential micronutrient for humans. While an extreme or absolute Zn deficiency in animals and humans is uncommon, a marginal Zn deficiency has been identified in various human population groups in less developed countries, where the diet is essentially composed of cereals and root crops, and in some industrialized countries.1–7Although inadequate dietary intakes of Zn contributes to the occurrence of Zn deficiencies in humans, various dietary factors which interfere with the absorption and/or utilization of Zn by humans are the most likely common causative factor.1 Antinutritional factors, such as phytate, in staple plant foods can reduce the Zn bioavailability in meals.1,8 Therefore, a low dietary Zn intake combined with poor dietary bioavailability have led to a Zn deficiency in some human populations. The removal or reduction of phytate by an enzyme treatment markedly improves dietary Zn absorption.9–11 Foods of animal origin contain unknown factors that promote Zn bioavailability.1,12,13 Studies have shown that the Zn bioavailability from staple plant food dietary sources can be improved by incorporating animal protein into a meal.13,14 However, the mechanism of this effect is not known. Due to the high costs and the complexities in determining the bioavailability of Zn in plant foods in human feeding trials, and because rat models are not ideal for determining Zn bioavailability to humans (i.e. rats are much more efficient at absorbing Zn from plant foods than humans are), it has become apparent that an in vitro method capable of monitoring Zn bioavailability from staple food crops would be a valuable tool towards improving the nutritional quality of plant foods. An in vitro digestion/Caco-2 cell culture model has been used to determine the Fe bioavailability in plant foods.15 Studies with this model have demonstrated that the Caco-2 cell formation of ferritin, the intracellular iron storage protein, occurs in response to iron uptake and can be used as a measure of cell-iron uptake.15–17 The use of ferritin as a marker for Fe uptake negates the need for isotopic labelling of the food samples and enables a high throughput in the in vitro system. Metallothioneins (MTs) are proteins which play an important role in the homeostasis of Zn and other metals. These proteins have an ability to bind metals and have been detected in a variety of mammalian cell types including intestinal cells.18,19 Therefore, the objective of the present study was to determine if cellular MT formation could be used as a proxy for Zn absorption in an in vitro digestion/Caco-2 cell culture model. As MT is not specific for Zn alone, if MT formation occurred, the initial Zn absorption information from the Caco-2 cell model will be validated by other available methods, such as the isotopic method.
2. Materials and methods
2.1 Chemicals, enzymes and hormones
Unless otherwise stated, all chemicals, enzymes, and hormones were purchased from Sigma Chemical Co. (St. Louis, MO‡).2.2 Zinc uptake solutions
The stock solution contained 100 mmol L−1 of Zn (as ZnCl2), dissolved in the uptake buffer. The uptake buffer was a minimum essential medium (GIBCO) supplemented with 10 mM PIPES (piperazine-N,N′-bis 2-ethanesulfonic acid), 1% antibiotic antimycotic solution (GIBCO), hydrocortisone (4 mg L−1), insulin (5 mg L−1), selenium (5 μg L−1), triiodothyroniine (34 μg L−1) and epidermal growth factor (20 μg L−1). Sufficient stock solution was then diluted with the uptake buffer to achieve 0, 2, 5, 10, 25, 50, 75, and 100 μmol L−1 of Zn in the uptake solutions.To prepare the solutions containing increasing amounts of phytic acid (PA), a stock ZnCl2 solution was first combined with PA to achieve Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA molar ratios of 1
PA molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1
0, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and 1
10, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. An uptake buffer was then added to dilute the mixture to a final concentration of 50 μmol L−1 of Zn in the uptake solutions.
20. An uptake buffer was then added to dilute the mixture to a final concentration of 50 μmol L−1 of Zn in the uptake solutions.
2.3 Zinc uptake experiments
In the experiments where ZnCl2 was added to the media, the experiments were replicated five times. Each experimental treatment was performed in triplicate for each replication of the experiment. The experiments involving PA were replicated four times in order to study the relationship between cellular Zn uptake and cellular MT formation, and each experimental treatment was performed in triplicate for each replication of the experiment. Replicates of each experiment were conducted on separate days. The maintenance of the Caco-2 cell culture and the seeding of the 6-well plates for the experiment were described previously by Glahn et al.15 On the day of the experiment, the cell medium was first removed from each culture well and the cell monolayers were rinsed twice with the uptake buffer. Each monolayer then received 2 mL of the Zn uptake solution. The cells were allowed to incubate with the Zn uptake solutions for 24 h in a 5% CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95% air incubator at 37 °C with constant humidity. To remove extracellular-bound Zn at the end of the 24 h incubation, the cell monolayers were rinsed twice with 2 ml of a chelating solution containing 2 mmol L−1 of Na2EDTA. After rinsing, 2 ml of deionized water was added to each cell culture well and the plate was placed in contact with a sonicating water bath at 4 °C for 15 min. The monolayers were then removed by scraping and cellular tissues were collected for analysis.
95% air incubator at 37 °C with constant humidity. To remove extracellular-bound Zn at the end of the 24 h incubation, the cell monolayers were rinsed twice with 2 ml of a chelating solution containing 2 mmol L−1 of Na2EDTA. After rinsing, 2 ml of deionized water was added to each cell culture well and the plate was placed in contact with a sonicating water bath at 4 °C for 15 min. The monolayers were then removed by scraping and cellular tissues were collected for analysis.
2.4 Intracellular Zn and metallothionien assay
The intracellular concentration of Zn was determined via inductively-coupled, argon-plasma emission spectrophotometry (ICPES) after dissolving 500 μL of the cellular materials in 3 mL of a 5% HNO3 solution.The intracellular concentration of metallothionein (MT) was determined using the cadmium/hemoglobin affinity assay described by Eaton and Toal20 This method determined the amount of soluble Cd which correlates to the amount of MT in the sample. A 200 μL aliquot of a 109Cd solution at pH 7.4, containing 2.0 μg of Cd mL−1 and 37 kBq of 109Cd mL−1 in a buffer of 10 mmol L−1 of Tris-HCl, was added to a 200 μL volume of the cellular materials, mixed and then incubated at 37 °C for 10 min. Following the incubation, the mixture received two separate additions of 100 μL of a 2% bovine hemoglobin solution. The hemoglobin solution removed non-bound 109Cd from the sample. Each addition was followed by heating the mixture in a boiling water bath for 2 min, cooling the mixture to room temperature and centrifuging at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× g for 10 min. Three hundred μL of clear supernatant was then collected in a radioassay vial and the radioactivity of 109Cd was determined in a gamma spectrophotometer.
000× g for 10 min. Three hundred μL of clear supernatant was then collected in a radioassay vial and the radioactivity of 109Cd was determined in a gamma spectrophotometer.
2.5 Food samples preparation for in vitro digestion
All procedures, preparation of the digestion solutions (pepsin, pancreatin, and bile extract) and the in vitro digestion were performed as previously described by Glahn et al.15,16 Briefly, 1.0 g of the food sample was used for each sample digestion. Samples were autoclaved for 15 min. The autoclaved food samples were then homogenized in a polytron homogenizer. The homogenate was frozen and then lyophilized to dryness.2.6 Statistical analysis
The results were analyzed using the GraphPad Prism software (GraphPad Software, San Diego, CA) and ANOVA, using the general linear models procedure from the SAS software (SAS Institute Inc. Cary, NC). Prior to analysis and if appropriate, data was log transformed to achieve equal variance. Tukey's test was used to compare the various means of each series of experiments. The significance was defined at P < 0.05 (values in the text are means ± SEM).3. Results
3.1 Zinc uptake by Caco-2 cells
When the cells were incubated with the uptake solutions, the concentration of Zn in the cells receiving 25, 50 or 75 μmol L−1 of the Zn uptake solution increased as the concentration of the uptake solution increased (Fig. 1). However, the cellular Zn concentration between the cells incubated with 2, 5 or 10 μmol L−1 of the Zn uptake solution did not differ from that of the cells receiving an uptake solution with no added Zn. Caco-2 cell Zn uptake is dependent on the Zn media concentration. Because the cellular Zn concentration below 10 μmol L−1 was similar to the untreated cells, Zn deficient conditions in the cell experiments have been attempted to see if it decreases Zn absorption from the untreated cells. The original Zn concentration in the media was 0.286 μg ml−1. Cellular Zn absorptions were not significantly lowered when the Zn media concentrations were reduced to about 67% and 50% of the original Zn media concentration (0.191 and 0.148 μg ml−1). Furthermore, the cells were dead when the Zn media concentrations were reduced to about 30% (0.094 μg ml−1). The cells also responded accordingly in the formation of MT when the Caco-2 monolayers were incubated with ZnCl2 solutions containing 25 to 75 μmol L−1 of Zn. Nevertheless, the MT concentrations in the cell, indexed by the concentration of Cd, was no different after incubation with the uptake solutions containing various lower levels of Zn (0 to 10 μmol L−1 of Zn).
 | ||
| Fig. 1 Cellular Zn concentrations and cellular metallothionein (i.e., Cd bound to metallothionein) concentrations of Caco-2, incubated in an uptake media with increasing Zn concentrations. * Measurement of the cellular Zn concentration was determined by ICPES spectrophotometry. Error Bar = ± SEM; n = 5. The bar values with no letters in common are significantly different (P < 0.05). ** Measurement of cellular metallothionien formation via the cadmium (Cd) binding assay. Error Bar = ± SEM; n = 5. The bar values with no letters in common are significantly different (P < 0.05). | ||
Pearson's correlation between the concentration of cellular Zn and the concentration of Cd bound by MT was 0.930 (Fig. 2).
 | ||
| Fig. 2 Correlation between total cellular Zn and cellular metallothionein (i.e., Cd bound to metallothionein). The cellular Zn concentration was determined by ICPES of acid digested cellular materials. Pearson's correlation = 0.930. | ||
3.2 Effect of phytic acid on Zn uptake by Caco-2 cells
Caco-2 cells were incubated with Zn uptake solutions containing a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA molar ratio of 1
PA molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Fig. 3 shows the concentrations of cellular Cd bound to MT incubated with Zn uptake solutions with or without the presence of PA. The cellular concentration of MT did not differ between the cells incubated with 2, 5 or 10 μmol L−1 of Zn with or without added PA. At 15, 20, 25, and 30 μmol L−1 of Zn and without added PA, the cellular concentration of MT increased in response to the increasing Zn concentration in the uptake media. However, PA additions greatly suppressed the cellular concentrations of MT that remained at a lower level, similar to the MT found in cells incubated with 2, 5 or 10 μmol L−1 of Zn (Fig. 3).
10. Fig. 3 shows the concentrations of cellular Cd bound to MT incubated with Zn uptake solutions with or without the presence of PA. The cellular concentration of MT did not differ between the cells incubated with 2, 5 or 10 μmol L−1 of Zn with or without added PA. At 15, 20, 25, and 30 μmol L−1 of Zn and without added PA, the cellular concentration of MT increased in response to the increasing Zn concentration in the uptake media. However, PA additions greatly suppressed the cellular concentrations of MT that remained at a lower level, similar to the MT found in cells incubated with 2, 5 or 10 μmol L−1 of Zn (Fig. 3).
 | ||
Fig. 3 The concentration of metallothionein (determined by a Cd binding assay) in Caco-2 cells incubated with Zn uptake solutions with or without added phytic acid. Phytic acid was added to the uptake solutions in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; Zn 10; Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA. PA represents phytic acid. (■) triplicate (□) duplicate. PA. PA represents phytic acid. (■) triplicate (□) duplicate. | ||
When the cells were incubated with uptake solutions containing 50 μmol L−1 of Zn with increasing concentrations of PA, the presence of PA significantly lowered the concentration of cellular Zn and MT. The MT concentration was significantly lowered in the cells exposed to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 Zn
5 Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA molar ratio. Further increases in PA did not lead to further decreases in cellular MT concentrations. The cellular Zn concentration was decreased as the Zn
PA molar ratio. Further increases in PA did not lead to further decreases in cellular MT concentrations. The cellular Zn concentration was decreased as the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA ratio increased. The MT concentration was decreased as the cellular Zn concentration decreased (Fig. 4).
PA ratio increased. The MT concentration was decreased as the cellular Zn concentration decreased (Fig. 4).
 | ||
Fig. 4 The concentration of metallothionein (determined by a Cd binding assay) in Caco-2 cells incubated with Zn uptake solutions containing increasing amounts of phytic acid. Phytic acid (PA) was added to the uptake solutions containing 50 μM of Zn to provide molar ratios of Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA from 1 to 20. Cellular Zn concentration was determined by ICPES of acid digested cellular materials (values are means ± SEM, n = 4). PA from 1 to 20. Cellular Zn concentration was determined by ICPES of acid digested cellular materials (values are means ± SEM, n = 4). | ||
3.3 Zn uptake in foods
In the presence of food, no significant MT formation above the baseline was found (Fig. 5).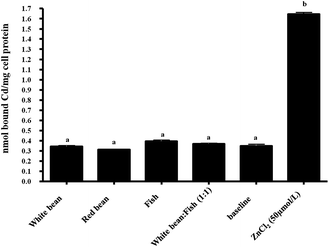 | ||
Fig. 5 The concentration of metallothionein (via a Cd binding assay) in Caco-2 cells incubated with either 50 μM of ZnCl2 or an in vitro digest of white beans, red beans, fish and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of white beans and fish. The baseline indicates the Caco-2 cell metallothionein levels without the addition of a food digest or ZnCl2 (values are mean ± SEM, n = 6). 1 mixture of white beans and fish. The baseline indicates the Caco-2 cell metallothionein levels without the addition of a food digest or ZnCl2 (values are mean ± SEM, n = 6). | ||
4. Discussion
The primary objective of the present study was to determine if MT formation by Caco-2 cells could serve as a measure of Zn uptake by the cells. If this is possible, then Caco-2 cell monolayers may be useful for measuring Zn bioavailability in foods. This method would have several advantages. First, it would negate the need for isotopic labelling of the foods to measure Zn uptake. Second, it would enable a high throughput of samples through an in vitro system, which is necessary for screening Zn bioavailability in food. Third, if MT is formed then we can be confident that uptake has occurred and not merely nonspecific surface binding. Finally, this method could be used simultaneously in an established in vitro model of Fe bioavailability in food, as sufficient cell material is available for the measurement of both Fe and Zn uptake in the conditions of this model.15 Iron and zinc deficiencies often occur simultaneously in many populations and hence the bioavailability of both Fe and Zn are of interest in nutritional interventions. In biofortification programs such as HarvestPlus, Fe and Zn are the primary minerals of interest.For Caco-2 cell monolayers to be a useful screening tool for Zn availability, several conditions must be possible. First, the cells must show responsiveness (i.e. Zn uptake) over the range of Zn concentrations that will be present during the in vitro digestion of foods. For staple foods such as beans, wheat and maize, Zn concentrations range between 30–100 μg g−1. Hence, if a typical 1 g sample is used in an in vitro digest volume of 15 mL, the Zn concentration would be in the range of 30–100 μmol L−1. In the present study, the Caco-2 cells that were exposed to a media containing Zn at a concentration of 100 μmol L−1 exhibited signs of toxicity from the added Zn. For the purposes of in vitro screening, this level for toxicity is not necessarily a problem as the concentration of food in the digest can be reduced to accommodate this limitation. Therefore, the next key factor to be determined is the initial detection limit of this method and to define the range of Zn concentrations that can be measured under these cell culture conditions.
In the present study we simply used ZnCl2 solutions to define the MT response range. The results of the studies where ZnCl2 was added to the cell culture media indicate that a range of 10–75 μmol L−1 is the working range for MT formation. This range would be sufficient for most foods that would be screened in this in vitro system. However, the key point to consider is that in the presence of foods, Zn uptake could be much lower depending on the interaction with compounds such as phytic acid within the food matrix.
In the presence of food, and using the defined conditions of our in vitro digestion/Caco-2 cell model, we found that no significant MT formation above the baseline occurred. This suggests that in the presence of a food matrix, the Caco-2 cell monolayer model may not be adequate to assess Zn uptake. As previously shown by Scarino et al., and Han et al., we observed that phytic acid inhibits Zn absorption and that Zn induces MT expression.21,22 To confirm this effect we reverted to more simple conditions of simply adding Zn and phytic acid at combinations that would be representative of food. Clearly, phytic acid is a strong inhibitor of Zn uptake and Zn-induced MT in vitro, and many of the foods that are of interest, such as staple food crops, will be high in phytic acid at molar Zn to PA ratios of 1 to 5 or higher. Our results show that under these conditions, no measurable Zn uptake occurs and hence, this model does not appear useful as a high throughput screening tool towards measuring Zn bioavailability from food. For example, at low Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA values (1
PA values (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), cellular uptake of Zn was almost completely inhibited and in foods the PA values will be even higher. This fact was clearly evident in Fig. 5, where no measurable Zn uptake could be demonstrated from bean samples. In the bean samples, Zn
1), cellular uptake of Zn was almost completely inhibited and in foods the PA values will be even higher. This fact was clearly evident in Fig. 5, where no measurable Zn uptake could be demonstrated from bean samples. In the bean samples, Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA ratios were 1
PA ratios were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 and 1
12 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 in red and white beans, respectively. Even the presence of fish had no significant effect on Zn uptake.
17 in red and white beans, respectively. Even the presence of fish had no significant effect on Zn uptake.
Previous studies have shown that other metals (e.g. copper) can stimulate MT expression in Caco-2 cells and suckling rat pups.21,23 In addition, dietary polyphenol (e.g. tannic acid) can also stimulate MT expression in Caco-2 cells.24 In order for MT to be a useful proxy to measure Zn absorption from food, it would have to be specific for Zn. This aspect must be considered and more ways are needed to ensure specific Zn-induced MT formation in future studies.
In summary, the results of this study suggest that the total cellular Zn concentration determined via ICPES and the MT method demonstrate good correlation under conditions where ZnCl2 is simply added to a cell culture media. However, in the presence of a real food matrix, little or no measurable Zn uptake occurs. In staple food crops, phytic acid is most likely the primary inhibitor of Zn uptake as it showed a strong effect under these cell culture conditions.
5. Conclusion
We conclude that under these experimental conditions, Caco-2 cells respond to Zn within a cell culture media but not to Zn from food. Alternative approaches need to be developed for in vitro assessments of Zn availability.Acknowledgements
The authors thank Mary Bodis, Yongpei Chang and Larry Heller for their excellent technical assistance. The research was supported by CGIAR HarvestPlus.References
- K. M. Hambidge, C. E. Casey and N. F. Krebs, in Trace Elements in human and animal nutrition, Mertz W. ed., Academic Press, Inc., New York, NY, 5th ed 1986, pp. 1137 Search PubMed.
- J. G. Reinhold, in Trace Minerals in Foods, Smith K. T. ed., Marcel Dekker, Inc., New York, NY and Basel, Switzerland, 1998, pp.155 Search PubMed.
- H. H. Sandstead, Zinc: essentiality for brain development and function, Nutrition Today, 1984, 19, 2630 CrossRef.
- R. S. Gibson, Nutr. Res. Rew., 1994, 7, 151–173 CAS.
- R. Shrimpton, SCN News., 1993, 9, 24–27 Search PubMed.
- R. S. Gibson, P. D. Smit Vanderkooy, A. C. MacDonald, A. Goldman, B. A. Ryan and M. A. Berry, Am. J. Clin. Nutr, 1989, 49, 1266–1273 CAS.
- K. M. Hambidge, Am. J. Clin. Nutr., 1997, 125, 1042S–1050S Search PubMed.
- N. R. Reddy, M. D. Pierson, S. K. Sathe and D. R. Salunkhe, in Phytates in Cereals and Legumes., CRC Press, Boca Raton, FL, 1989 Search PubMed.
- V. V. Agte, M. K. Gokhale and S. A. Chiplonkar, Int. J. Food Sci. Technol., 1997, 32(1), 29–32 CAS.
- J. R. Zhou, E. J. Fordyce, V. Raboy, D. B. Dickinson, M. S. Wong, R. A. Burns and J. W. Erdman, J. Nutr., 1992, 122(12), 2466–2473 CAS.
- M. Turk and A. S. Sandberg, J. Cereal Sci., 1992, 15, 281–294 CrossRef CAS.
- R. M. Welch, W. A. House, in Factors affecting the bioavailability of mineral nutrients in plant foods, in Crops as Sources of Nutrients for Humans, Welch R M and Gabelman W H ed., American Society of Agronomy, Madison, WI, 1984, pp. 37–54 Search PubMed.
- J. R. Hunt, G. I. Lykken and L. K. Mullen, Nutr. Res., 1991, 11(5), 413–418 CrossRef CAS.
- B. Sandstrom, A. Almgren, B. Kivisto and A. Cederblad, J. Nutr., 1989, 119(1), 48–53 CAS.
- R. P. Glahn, O. A. Lee, A. Yeung, M. I. Goldman and D. D. Miller, J. Nutr., 1998, 128(9), 1555–1561 CAS.
- R. P. Glahn, Z. Cheng, R. M. Welch and G. B. Gregorio, J. Agric. Food Chem., 2002, 50(12), 3586–3591 CrossRef CAS.
- C. K. Yeung, D. D. Miller, Z. Cheng and R. P. Glahn, J. Food Sci., 2005, 70(3), S199–S203 CrossRef CAS.
- M. A. Dunn, T. L. Blalock and R. J. Cousins, Proc. Soc. Exp. Biol Med., 1987, 185, 107–119 CAS.
- M. P. Richards and R. J. Cousins, Biochem. Biophys. Res. Commun., 1977, 75(2), 286–294 CrossRef CAS.
- D. L. Eaton and B. F. Toal, Toxicol. Appl. Pharmacol., 1982, 66(1), 134–142 CrossRef CAS.
- M. L. Scarino, R. Poverini, G. D. Lullo and G. Bises, Biochemical Society Transactions., 1991, 19, 283S CAS.
- O. Han, M. L. Failla, A. D. Hill, E. R. Morris, J. C. Smith and JR., J. Nutrition, 1994, 124, 580–587 CAS.
- K. A. Bauerly, S. L. Kelleher and B. Lonnerdal, J. Nutr. Biochem., 2004, 15, 155–162 CrossRef CAS.
- K. Sreenivasulu, R. Pullakhandam and K. N. Madhavan, J. Food Sci., 2010, 75(4), H123–H12 CrossRef CAS.
Footnotes |
| † This author performed this work while working at Cornell University and before he joined the U.S. Food and Drug Administration. |
| ‡ Mention of a trademark, proprietary product or vendor does not constitute a guarantee or warranty of the product by the United States Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that may also be suitable. |
| This journal is © The Royal Society of Chemistry 2012 |
