Urinary 6-sulfatoxymelatonin and total antioxidant capacity increase after the intake of a grape juice cv. Tempranillo stabilized with HHP†
David
González-Flores‡
a,
Esther
Gamero‡
b,
María
Garrido
a,
Rosario
Ramírez
b,
Daniel
Moreno
b,
Jonathan
Delgado
b,
Esperanza
Valdés
b,
Carmen
Barriga
a,
Ana B.
Rodríguez
*a and
Sergio D.
Paredes
a
aDepartment of Physiology (Neuroimmunophysiology and Chrononutrition Research Group), Faculty of Science, University of Extremadura, 06006, Badajoz, Spain. E-mail: moratino@unex.es; Fax: +34 924 289 388; Tel: +34 924 289 300, Ext.: 89035
bTechnological Institute of Food and Agriculture of Extremadura (INTAEX), 06071, Badajoz, Spain
First published on 28th October 2011
Abstract
Red grapes contain elevated amounts of antioxidant compounds (polyphenols) that may potentially prevent cell aging, cardiovascular disease and oxidation-related disorders. Since functional drinks are presently one of the most dynamic sectors of the market, the present work was aimed at evaluating the possible antioxidant effect of an experimental grape juice in terms of urinary 6-sulfatoxymelatonin (aMT6-s) and total antioxidant capacity in young (20 ± 10 yr-old), middle-aged (45 ± 10 yr-old) and elderly (75 ± 10 yr-old) individuals. Grapes (Vitis vinifera cv. Tempranillo) were de-stemmed, racked and pressed. The juice was subsequently stabilized by high hydrostatic pressure (HHP). Participants consumed 200 mL of grape juice twice a day (as the lunch and dinner desserts) for 5 days. First-void morning urines were collected before treatment (basal values), the day immediately after the last ingestion of juice (assay), and one day afterwards (post-assay). aMT6-s and total antioxidant capacity were quantified using commercial ELISA and colorimetric assay kits, respectively. The intake of grape juice cv. Tempranillo induced a significant increase of urinary aMT6-s and total antioxidant capacity in the three groups of age analyzed as compared to their corresponding basal and post-assay values. These functional/nutraceutical properties may be of interest for a prospective commercialization of the grape juice. The novel technology used for juice stabilization may be suitable for introducing into the market a product with high sensory and nutritional quality, as it has been shown in this study.
Introduction
The relationship between diet and health has led to intense research into bioactive compounds in foods. Many studies indicate that constituents of fruits and vegetables are protective against a variety of diseases.1 The health-promoting effects of plant foods and plant-derived beverages have been traditionally attributed to some chemical constituents present in various plant tissues, i.e., phenylpropanoids, isoprenoids, and alkaloids.2–4 These phytochemicals can act as dietary therapeutics or nutraceuticals and, therefore, by virtue of their content, some plant foods and beverages can be considered as functional foods and beverages, i.e. products consumed as part of a normal diet that may provide health benefits beyond basic nutritional functions.5,6A growing body of knowledge has shown that numerous valuable compounds with nutraceutical properties are present in grapes (Vitis vinifera).7 Although nutritional characteristics of grapes have been mostly associated with the afore-mentioned phytochemicals, especially phenylpropanoids, the recent discovery of the indoleamine melatonin in different grapevine cultivars8–10 has added new elements to the plethora of health benefits associated to the consumption of these fruits.
Melatonin, like its precursor, the essential amino acid tryptophan, exerts a sleep enhancing effect and improves sleep and certain pathological conditions associated with sleep. Indeed, age-related disturbances in the sleep-wake and temperature rhythms have been correlated with age-related reductions in the amplitude of the nocturnal melatonin peak.11,12Melatonin also possesses immunoregulatory properties and is able to stimulate antioxidant enzymes, as well as inhibiting the prooxidative enzyme nitric oxide synthase, and diminishing free radical formation at the mitochondrial level or to synergize with other antioxidants to protect against oxidative stress.13 This has led some to speculate that the intake of foodstuffs rich in melatonin and its indolic precursors (tryptophan and serotonin) may represent a dietetic tool for counteracting oxidative stress and sleep disorders. This is the case of Jerte Valley cherries, whose consumption, either fresh or as a nutraceutical product, has been reported to exert positive effects on nocturnal rest and to elevate the levels of 6-sulfatoxymelatonin (aMT6-s) and antioxidant capacity in the urine of middle-aged and elderly subjects.14,15 Little is known, however, about grapes or grape-derived beverages, including juice. Hence, the aim of the present work was to evaluate the possible antioxidant effect of diets supplemented with a grape juice in young, middle-aged, and elderly individuals. High hydrostatic pressure (HHP) was assayed for the stabilization of the grape juice, since this technology has been proved to minimize the inclusion of chemical additives in the product and to have no effect on its physicochemical characteristics and nutritional value, contributing to preserve the quality and beneficial properties of the grape juice.
Results and discussion
Thermal stabilization has been a common process employed in the elaboration of grape juice. Currently, alternative techniques to the use of temperature are being actively researched due to the changes in physicochemical and organoleptic properties that normally appear in grape-derived products after thermal stabilization. In the present work, a technique using HHP was implemented with the purpose of stabilizing a grape juice cv. Tempranillo. Results of analysis of HHP-stabilized grape juice parameters are shown in Table 1. Microbiological assay revealed that this technique reduced microbiological counts of mesophiles, psychotrophes, Enterobacteriaceae, molds and yeasts under the detection limit of the method. Furthermore, it was observed that the juice was free of pathogens Listeria monocytogenes and Salmonella after HHP application.| Parameter | Mean |
|---|---|
| a Total acidity (g L−1 of tartaric acid). b Total polyphenols (mg L−1 of gallic acid). c Total anthocyans (malvidin glycoside). d Catechins (mg L−1D-catechin). | |
| pH | 3.7 |
| Acidity (g L−1) | 5.33 |
| Soluble solids (°Brix) | 22.41 |
| Total polyphenols (mg L−1) | 679.79 |
| Total anthocyans (mg L−1) | 407.37 |
| Total tannins (mg L−1) | 362.05 |
| Catechins (mg L−1) | 437.10 |
| Color index | 3.33 |
| Color tonality | 0.52 |
Grape chemistry is rather complex and over 1600 compounds have been identified in the genus Vitis, arising from the three main classes of natural products, phenylpropanoids, isoprenoids and alkaloids, widely distributed both in plant foods and medicinal herbs.16,17 Recently, the occurrence of melatonin in all grape tissues (skin, flesh, and seed) at two different phenological stages, pre-véraison and véraison, has been reported.10 However, studies on the bioavailability of melatonin contained in these berries and the possible impact that their intake may have on organismal health have not been tackled. Here, a significant (p < 0.05) increase in urinary aMT6-s levels in young, middle-aged and elderly volunteers with respect to the basal values was shown (Fig. 1A, 1B, and 1C). Interestingly, increases reached by middle-aged and elderly individuals were higher than that found in the young group. This is indirect evidence for a rise in circulating melatonin levels as a result of the intake of the grape juice. In fact, urinary aMT6-s, the major urinary metabolite of melatonin, accurately reflects nocturnal plasma melatonin.18,19 These results are consistent with previous studies where associations between the consumption of vegetables and fruits that are high in melatonin content and elevated melatonin levels in both blood and urine have been established.20,21 Particularly, Reiter et al.21 showed that the consumption of walnuts, which are rich in melatonin, provoked a threefold increase in circulating melatonin levels and also improved serum antioxidant capacity measured in trolox equivalents. Moreover, the consumption of cherries, either fresh or as a nutraceutical cherry-based beverage, has been shown to improve sleep as well as increasing total antioxidant capacity and aMT6-s levels in first-void urines of middle-aged and elderly humans.14,15
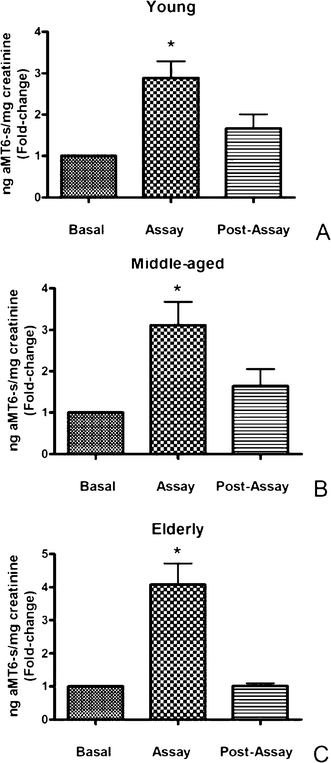 | ||
| Fig. 1 Effect of the intake of HHP-stabilized grape juice on urinary aMT6-s levels expressed as nanogram aMT6-s per milligram creatinine in basal (urine samples obtained before the intake of grape juice), assay (urine samples taken after 5 days of intake of 200 mL juice twice a day) and post-assay (urine samples taken 1 day afterwards) conditions in young (A), middle-aged (B) and elderly (C) participants. Results are expressed as fold-change over the basal level (experimental/basal). Each value represents the mean ± SD of ten determinations carried out in duplicate. *p < 0.05 with respect to basal and post-assay values. | ||
Fig. 2 (A, B, and C) shows the results of the urine antioxidant capacity before and after HHP-stabilized grape juice consumption. Juice intake provoked a significant (p < 0.05) increase in urinary antioxidant levels in young, middle-aged and elderly volunteers with respect to the basal and post-assay values. At this respect, numerous investigators, on the basis of their experimental results, have concluded that free radical mutilation of essential molecules are related to deteriorative cellular and organismal changes associated with aging and also with the development of a variety of age-related diseases.22,23 Thus, consuming the juice tested in the present study may have a protective effect against oxidation. Polyphenols may be responsible of this rise. Particularly, daily consumption of grape juice (10 mL kg−1 body weight) for 2 weeks resulted in an increased resistance of LDL to ex vivooxidation, comparable to the value obtained after α-tocopherol (400 IU) supplementation.24 Also, daily intake of grape juice (125 mL) for 1 week significantly reduced LDL oxidazibility and increased plasma antioxidant capacity, measured 1 h after grape juice supplementation, as ferric-reduced antioxidant potential (FRAP).25 In a short-term study, the acute intake of a phenolic-rich juice (400 mL), with grapes as a major ingredient, improved the antioxidant status in healthy subjects, determined both in serum and urine by FRAP.26 In the same work, the authors showed that the phenolic compounds of the juice were bioavailable, as revealed by the increase of phenolics able to bind the lipid fraction of serum and their rise in urinary excretion, with a maximum reached 2 h after consumption. Since it is known that increased levels of circulating melatonin, directly by exogenous administration or indirectly by introducing in the diet vegetables rich in this molecule, enhances the individual's antioxidant status,14,15,21,27 contribution of this indoleamine to the observed antioxidant elevation cannot be ruled out.
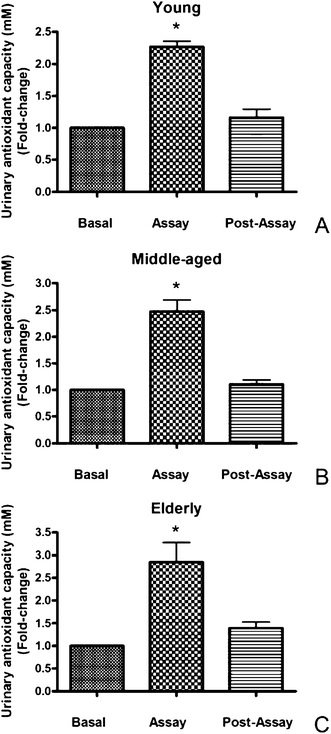 | ||
| Fig. 2 Effect of the intake of HHP-stabilized grape juice on urinary antioxidant capacity (mM) measured in basal (urine samples obtained before the intake of grape juice), assay (urine samples taken after 5 days of intake of 200 mL juice twice a day) and post-assay (urine samples taken 1 day afterwards) conditions in young (A), middle-aged (B) and elderly (C) participants. Results are expressed as fold-change over the basal level (experimental/basal). Each value represents the mean ± SD of ten determinations carried out in duplicate. *p < 0.05 with respect to basal and post-assay values. | ||
Experimental
Elaboration of grape juice
Red grapes cv. Tempranillo were used for the elaboration of the juice. Grapes were harvested from a local vineyard located at Centro de Investigación Finca “La Orden-Valdesequera” (Junta de Extremadura). Grapes were washed with sulphited water and manually selected, in order to remove leaves and bunches with poor phytosanitary quality. Once selected, grapes were de-stemmed and racked, adding L-ascorbic acid (1g/Kg grapes) to prevent oxidation. The obtained paste was then soaked in hot conditions (40 °C for 10 min) in the presence of pectolytic enzymes (2 g per 100 kg grapes) with the purpose of increasing the extraction of polyphenolic compounds. After completing the soaking step, the paste was cooled and then lightly pressed (1 bar for 30 s) in a pneumatic press (Willmes D-68623, Lampertheim, Germany), being obtained a juice that was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min at 4 °C (Beckman Coulter Allegra 25, Izasa, Barcelona, Spain). Finally, the supernatant was bottled and sealed in low-density polyethylene containers.
000 rpm for 5 min at 4 °C (Beckman Coulter Allegra 25, Izasa, Barcelona, Spain). Finally, the supernatant was bottled and sealed in low-density polyethylene containers.
High hydrostatic pressure method
Grape juice was subjected to a high hydrostatic pressure (HHP) treatment, 6000 bar for 7 min (Hyperbaric Wave 6000/55), in order to reach the microbiological stabilization of the product. Soluble solid contents (Brix) were determined by refractometry (ATR ST plus, Schmidt + Haensch GmbH & Co., Berlin, Germany). Both pH and acidity were determined by an automatic Crison Micro TT according to the CEE official methodology.28 Total polyphenols, anthocyanins and tannins were determined according to Iland et al.,29tannins following the method created by the Australian Wine Research Institute30 and catechins according to Swain and Hillis.31 Color intensity and tonality of the grape juice were evaluated using the method of Riberau-Gayon.32 The complete process is summarized in Chart 1.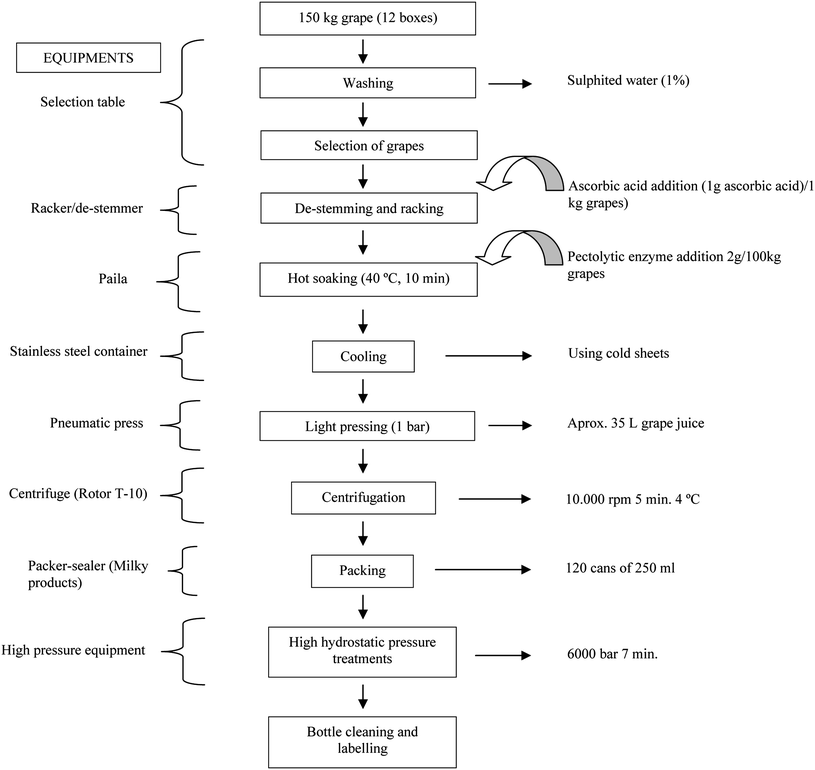 | ||
| Chart 1 Elaboration of the grape juice stabilized with HHP. | ||
Microbiological analysis
For the determination of sanitary state and life of the product, a microbiological study (mesophiles, psychotrophes, yeasts, molds, and Enterobacteriaceae) was performed after the HHP treatment. A new analysis was carried out 25 days afterwards. The methods used for the quantification of microorganisms were those established by ISO standards 4833 (July 1991): Horizontal method for the enumeration of microorganisms at 30 °C (Mesophiles); ISO 4833 (July 1991),33 with a modification in incubation time (from 3 to 5 days) and temperature (from 30 to 17 °C) for the enumeration of psychotrophes; ISO 7954 (August 1988) horizontal method for the enumeration of molds and yeasts, replacing cell medium YGC to CGA;34 horizontal method for the enumeration of Enterobacteriaceae without resuscitation, based on the ISO 7402 standard (December 1993);35ISO 11290 (December 1997) horizontal method for the enumeration and detection ofListeria monocytogenes;36ISO 6579 (December 1993) horizontal method for the enumeration and detection ofSalmonella.37Subjects and experimental design
The study was carried out in young (20 ± 10 yr-old, N = 6), middle-aged (45 ± 10 yr-old, N = 6) and elderly (75 ± 10 yr-old, N = 6) participants and it was approved by the Ethical Committee of the University of Extremadura (Badajoz, Spain), in accordance with the Declaration of Helsinki, the Council of Europe, and the Universal Declaration of UNESCO on human rights, biomedicine, and human genome. Each volunteer was ascertained to be in good health by means of their medical history and a clinical examination including routine laboratory tests and screening. They consumed 200 mL of grape juice twice a day (as the lunch and dinner desserts) for 5 days. First-void morning urines were collected before treatment (basal values), the immediate day after the last ingestion of grape juice (assay), and 1 day afterwards (post-assay).Determination of aMT6-s levels
For the quantification of urinary aMT6-s, a commercial ELISA kit (IBL, Hamburg, Germany) was used according to the manufacturer's instructions. To adjust for variation in the dilution of urine, aMT6-s concentrations were expressed as urinary aMT6-s/urine creatinine; creatinine concentration was determined by means of the Jaffe test, as described elsewhere.14,15Urine total antioxidant capacity
Urine total antioxidant capacity was evaluated by means of a colorimetric assay kit (Cayman, Michigan, USA), according to the manufacturer's instructions. This assay relies on the ability of antioxidants in the sample to inhibit the oxidation of ABTS® (2,2′-azino-di-[3-ethylbenzthiazoline sulphonate]) to ABTS®●+ by metmyoglobin. The capacity of the antioxidants in the sample to prevent ABTS®oxidation was compared with that of Trolox, a water-soluble tocopherol analogue, and quantified as millimolar Trolox equivalents.Statistical analysis
Friedman and Kruskal-Wallis non-parametric tests followed by Dunns' multiple comparison tests were used to analyze the results. Each value represents the mean ± SD of the number of determinations carried out in duplicate. The degree of significance was set at p < 0.05. All analyses were performed using GraphPad Prism® (version 5.0, 2007, San Diego, CA).Conclusions
The ingestion of 200 mL of grape juice twice a day stabilized with HHP significantly increased urinary aMT6-s and total antioxidant capacity in young, middle-aged and elderly individuals. HHP decreased the initial microbial charge with no alteration on the physicochemical properties of the product. The novel technology used for juice stabilization may be suitable for introducing into the market a product with high sensory, nutritional quality, and nutraceutical properties.Acknowledgements
We are indebted to the volunteers in this study for their outstanding commitment and cooperation. This investigation was supported by a research grant from INTERREG 0318-RITECA-4E; Program POCTEP, 053/09. Sergio D. Paredes was the beneficiary of a grant from Consejería de Economía, Comercio e Innovación-Fondo Social Europeo (Junta de Extremadura, REI09009).Notes and references
- C. K. B. Ferrari, Biogerontology, 2004, 5, 275–289 CrossRef CAS.
- P. J. Facchini, Annu. Rev. Plant Physiol. Plant Mol. Biol., 2001, 52, 29–66 CrossRef CAS.
- S. A. Holstein and R. J. Hohl, Lipids, 2004, 34, 293–309 CrossRef.
- M. Iriti and F. Faoro, Curr. Top. Nutr. Res., 2004, 2, 47–65 CAS.
- D. Heber, Nutr., 2004, 134, 3175S–3176S CAS.
- J. Zhao, Recent Pat. Biotechnol., 2007, 1, 75–97 CrossRef CAS.
- M. Iriti and F. Faoro, Med. Hypotheses, 2006, 67, 833–838 CrossRef CAS.
- M. Iriti, M. Rossoni and F. Faoro, J. Sci. Food Agric., 2006, 86, 1432–1438 CrossRef CAS.
- S. D. Paredes, A. Korkmaz, L. C. Manchester, D. X. Tan and R. J. Reiter, J. Exp. Bot., 2009, 60, 57–59 CrossRef CAS.
- S. Vitalini, C. Gardana, A. Zanzotto, P. Simonetti, F. Faoro, G. Fico and M. Iriti, J. Pineal Res., 2011, 51 DOI:10.1111/j.1600-079X.2011.00893.x.
- S. D. Paredes, M. P. Terron, V. Valero, C. Barriga, R. J. Reiter and A. B. Rodriguez, Basic Clin. Pharmacol. Toxicol., 2007, 100, 258–268 CrossRef CAS.
- S. D. Paredes, A. M. Marchena, I. Bejarano, J. Espino, C. Barriga, R. V. Rial, R. J. Reiter and A. B. Rodríguez, J. Gerontol. A. Biol. Sci. Med. Sci., 2009, 64, 340–350 CrossRef.
- S. D. Paredes and R. J. Reiter, Cell Membr. Free Radic. Res., 2010, 2, 99–111 Search PubMed.
- M. Garrido, J. Espino, D. González-Gómez, M. Lozano, J. Cubero, A. F. Toribio-Delgado, J. I. Maynar-Mariño, M. P. Terrón, J. L. Muñoz, J. A. Pariente, C. Barriga, S. D. Paredes and A. B. Rodríguez, Eur. J. Clin. Nutr. Metab., 2009, 4, 321–323 CrossRef.
- M. Garrido, S. D. Paredes, J. Cubero, M. Lozano, A. F. Toribio-Delgado, J. L. Muñoz, R. J. Reiter, C. Barriga and A. B. Rodríguez, A. Biol. Sci. Med. Sci., 2010, 65, 909–914 Search PubMed.
- J. M. Pezzuto, J. Agric. Food Chem., 2008, 56, 6777–6784 CrossRef CAS.
- M. Iriti and F. Faoro, Nat. Prod. Commun., 2009, 4, 611–634 CAS.
- G. Klante, T. Brinschwitz, K. Secci, F. Wollnik and S. Steinlechner, J. Pineal Res., 1997, 23, 191–197 CrossRef CAS.
- S. Oba, K. Nakamura, Y. Sahashi, A. Hattori and C. Nagata, J. Pineal Res., 2008, 45, 17–23 CrossRef CAS.
- C. Nagata, Y. Nagao, C. Shibuya, Y. Kashiki and H. Shimizu, Cancer Epidemiol. Biomarkers Prev., 2005, 14, 1333–1335 CAS.
- R. J. Reiter, L. C. Manchester and D. X. Tan, Nutrition, 2005, 21, 920–924 CrossRef CAS.
- D. Harman, Ann. N. Y. Acad. Sci., 2006, 1067, 10–21 CrossRef CAS.
- F. L. Muller, M. S. Lustgarten, Y. Jang, A. Richardson and H. Van Remmen, Free Radical Biol. Med., 2007, 43, 477–503 CrossRef CAS.
- D. J. O'Byrne, S. Devaraj, S. M. Grundy and I. Jialal, Am. J. Clin. Nutr., 2002, 76, 1367–1374 CAS.
- A. P. Day, H. J. Kemp, C. Bolton, M. Hartog and D. Stansbie, Ann. Nutr. Metab., 1997, 41, 353–357 CrossRef CAS.
- J. García-Alonso, G. Ros, M. L. Vidal-Guevara and M. J. Periago, Nutr. Res., 2006, 26, 330–339 CrossRef.
- S. D. Paredes, M. P. Terron, A. M. Marchena, I. Bejarano, J. Espino, C. Barriga, R. V. Rial, R. J. Reiter and A. B. Rodríguez, Mol. Cell. Biochem., 2007, 304, 305–314 CrossRef CAS.
- CEE, “Reglamento n° 2676/90 de la Comisión, de 17 de Septiembre de 1990, por el que se determinan los métodos de análisis comunitarios aplicables en el sector del vino en Métodos de análisis comunitarios aplicables en el sector del vino”. (DOCE n° 272 de 3 de Octubre de 1990), 1990 Search PubMed.
- P. Iland, N. Bruer, G. Edwards, S. Weeks and E. Wikes, Chemical Analysis of Grapes and Wine: Techniques and Concepts, Patrick Iland, Campelltown, South Australia, 2004 Search PubMed.
- C. J. Sarneckis, P. A. Smith, R. G. Dambergs, Jones Phillip, Markus J Herderich and M. Mercurio, Australian J. Grape Wine Res., 2006, 12, 39–49 CrossRef CAS.
- T. Y. Swain and W. Hillis, J. Sci. Food Agric., 1959, 10, 63–68 CrossRef CAS.
- P. Ribereau-Gayon, Y. Glories, A. Maujean, and D. Dubourdieu, Phenolic compound, in Handbook of Enology: The Chemistry of Wine Stabilization and Treatments, 2000, vol. 2, pp. 129–187 Search PubMed.
- ISO 4833, Microbiologie. Directives générales pour le dénombrement des micro-organismes. Méthode par comptage des colonies obtenues à 30 °C, 1991 Search PubMed.
- ISO 7954. Microbiologie. Directives générales pour le dénombrement des levures et moisissures. Technique par comptage des colonies obtenues à 25 °C, 1988 Search PubMed.
- ISO 7402. Microbiologie. Directives générales pour le dénombrement sans revivification des Enterobacteriaceae. Technique NPP el méthode par comptage des colonies, 1993 Search PubMed.
- ISO 11290. Microbiologie des aliments. Méthode horizontale pour la recherche et le dénombrement Listeria monocytogenes, 1997 Search PubMed.
- ISO 6579. Microbiologie. Directives générales concernant les méthodes de recherche des Salmonella, 1993 Search PubMed.
Footnotes |
| † Author disclosure statement: No competing financial interests exist. |
| ‡ Both authors contributed equally to this work |
| This journal is © The Royal Society of Chemistry 2012 |
