Impact of phloretin and phloridzin on the formation of Maillard reaction products in aqueous models composed of glucose and L-lysine or its derivatives
Jinyu
Ma
a,
Xiaofang
Peng
a,
Kwan-Ming
Ng
b,
Chi-Ming
Che
b and
Mingfu
Wang
*a
aSchool of Biological Sciences, Department of Chemistry, University of Hong Kong, Pokfulam Road, Hong Kong, P. R. China. E-mail: mfwang@hku.hk; Fax: +852-22990347; Tel: +852-22990338
bDepartment of Chemistry, University of Hong Kong, Pokfulam Road, Hong Kong, P. R. China
First published on 8th December 2011
Abstract
In the present study, the effects of phloretin and phloridzin on the formation of Maillard reaction products in a lysine–glucose model with different reactant ratios were systematically investigated. In terms of the formation of Maillard-type volatiles, phloretin and phloridzin treatmen could significantly reduce their generation, where the effects depend on the ratio of lysine to glucose used in the model systems. Phloretin and phloridzin could also affect the colour development of Maillard reactions; especially phloretin, which could significantly promote the formation of brown products in the system with the lowest ratio of lysine to glucose. Based on the carbon module labelling (CAMOLA) technique and HPLC-DAD-ESI/MS analysis, it was found that phloretin and phloridzin could actively participate in the Maillard reaction and directly react with different reactive carbonyl species. The effect of phloretin and phloridzin treatment in both Nα-acetyllysine–glucose (AC–glu), and N-acetyl-gly-lys methyl ester acetate salt–glucose (AG–glu) model systems, which are close to the Maillard reactions occurring in real food, where the free amino groups of lysine residues were considered as the reactive site, were further investigated. Similar impacts on the formation of Maillard-type volatiles and brown products as in the lysine–glucose models were observed which can also be explained by the capability of phloretin and phloridzin to quench sugar fragments formed in these model reactions.
Introduction
The Maillard reaction plays an important role in food science due to its ubiquitous impact on food flavour, colour, nutritional value and safety during thermal processing and food storage.1–3 The Maillard reaction is initiated by the reaction of reducing sugars with substances containing free amino groups, leading to the formation of a group of complex and heterogeneous products, including volatile and non-volatile compounds.4–6 The nature of Maillard reaction depends on many factors such as pH, temperature, pressure, moisture, and reactants in the reaction systems.7–9 Different reducing sugars, amino acids, and their combinations lead to different reaction kinetics with the formation of different classes of products. The ratio of reducing sugars to amino acids has been suggested as an important factor in determining the reaction rate of Maillard reactions. For example, several studies have investigated the effects of the reactant ratio on the formation of volatiles in a cysteine–ribose model system,10,11 and colour development in a glucose–glycine model system.12,13 Some studies have also demonstrated significant effects of several phenolic compounds on the formation of Maillard reaction products. For example, Fujiwara et al.14 demonstrated that low concentrations of catechol compounds inhibited the formation of CML [Nε-(carboxymethyl)lysine, a major antigenic AGE] while high concentrations of catechol compounds enhanced the formation of CML by producing hydrogen peroxide. More recently, several phenolic compounds were found to significantly impact the formation of flavour compounds. It was reported that the addition of epicatechin could inhibit the thermal development of aroma compounds (i.e., Maillard reaction products) formed during ultra high-temperature (UHT) processing, low-heat processing and storage.15,16 In other studies using different Maillard model systems, epicatechin was demonstrated to directly quench one key class of Maillard intermediate products, a reactive carbonyl species, thus reducing the formation of pyrazine, pyridines and furan derivatives in these systems.17–19Phloretin and phloridizin are two polyphenols found in apples. Recently they were demonstrated to effectively trap methylglyoxal (MGO) and glyoxal (GO), two reactive carbonyl compounds under physiological conditions (pH 7.4, 37 °C). It was reported that more than 80% MGO was trapped within 10 min, and 68% GO was trapped within 24 h by phloretin while phloridzin also had strong trapping efficiency by quenching more than 70% MGO and 60% GO within 24 h.20 However little is known whether phloretin and phloridizin can affect Maillard flavour and colour formation under thermal conditions. In this study, these two phenolic compounds have been adopted to investigate their influence on the Maillard reaction in lysine–glucose (lys–glu), Nα-acetyllysine–glucose (AC–glu), and N-acetyl-gly-lys methyl ester acetate salt–glucose (AG–glu) model systems. We compared the volatile compound formation and colour development in these model systems with or without the addition of phloretin or phloridzin. In addition, the probable mechanism of action, hypothetically, the scavenging of reactive sugar fragments, which are key Maillard precursors or intermediate products, was subsequently elucidated via HPLC-DAD-ESI/MS analysis with a CAMOLA technique.
Materials and methods
Materials
D-Glucose, D-[13C6]-glucose (99% enrichment), L-lysine (L-lys), Nα-acetyllysine (AC-lys), N-acetyl-gly-lys methyl ester acetate salt (AG-lys), n-dodecane, HPLC-grade acetonitrile, phosphate buffered saline (PBS, 0.1 M at pH 7.4), phloretin (Ph) and phloridzin (Pz) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).Preparation of reaction mixtures
D-Glucose (0.45 M and 0.90 M), D-[13C6]-glucose (0.90 M), L-lys (0.90 M, 0.45 M and 0.15 M), AC–lys (0.45 M) and AG–lys (0.45 M) were prepared separately in PBS. Phloretin and phloridzin were individually dissolved in methanol to a concentration of 0.15 M.To study the effects of phloretin/phloridzin on the formation of Maillard reaction products in the lysine–glucose model, three reactant ratios: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were used. For control samples, 0.1 mL each of 0.15 M, 0.45 M and 0.90 M L-lysine was mixed with an equal volume of 0.45 M D-glucose in 1 mL ampoules and 0.8 mL of PBS was then added. For the phloretin or phloridzin treated samples, the ampoules were substituted by ones prefilled with phloretin or phloridzin [0.10 mL of phloretin or phloridzin solution (0.15 M) was pipetted into 1 mL ampoules, individually, and dried by nitrogen blowing]. Subsequently, the ampoules were sealed with a cross-fire torch. Samples containing phloretin or phloridzin alone were also prepared to see whether these two compounds may bring about colour changes themselves under heating conditions. For AC–glu and AG–glu models, 0.1 mL each of 0.45 M AC–lys and 0.45 M AG–lys was mixed with an equal volume of 0.45 M D-glucose and 0.8 mL of PBS in 1 mL ampoules prefilled with or without phloretin or phloridzin. Subsequently, the ampoules were sealed with a cross-fire torch. For CAMOLA reactions, the sugar solution was substituted by a 50% labelled glucose solution prepared by mixing equal volumes of 0.90 M glucose and 0.90 M D-[13C6]-glucose.
1 were used. For control samples, 0.1 mL each of 0.15 M, 0.45 M and 0.90 M L-lysine was mixed with an equal volume of 0.45 M D-glucose in 1 mL ampoules and 0.8 mL of PBS was then added. For the phloretin or phloridzin treated samples, the ampoules were substituted by ones prefilled with phloretin or phloridzin [0.10 mL of phloretin or phloridzin solution (0.15 M) was pipetted into 1 mL ampoules, individually, and dried by nitrogen blowing]. Subsequently, the ampoules were sealed with a cross-fire torch. Samples containing phloretin or phloridzin alone were also prepared to see whether these two compounds may bring about colour changes themselves under heating conditions. For AC–glu and AG–glu models, 0.1 mL each of 0.45 M AC–lys and 0.45 M AG–lys was mixed with an equal volume of 0.45 M D-glucose and 0.8 mL of PBS in 1 mL ampoules prefilled with or without phloretin or phloridzin. Subsequently, the ampoules were sealed with a cross-fire torch. For CAMOLA reactions, the sugar solution was substituted by a 50% labelled glucose solution prepared by mixing equal volumes of 0.90 M glucose and 0.90 M D-[13C6]-glucose.
The sealed samples were heated in an oil bath at 140 °C for 30 min. After heating, the samples were immediately removed, cooled in an ice–water bath, and prepared for further analysis.
Sample preparation for gas chromatography analysis
Head-space solid phase micro-extraction (HS-SPME) was applied to absorb the Maillard-type volatile compounds generated in these model reactions according to the procedures published by Ibañez et al.21 Briefly, the total reaction mixture (1.0 mL) was transferred to a headspace vial (14 mL) and spiked with 10 μL of internal standard (n-dodecane, 1.0%). The vials were sealed with a screw cap fitted with Tuf-Bond Teflon and incubated in a water-bath (38 °C) for 10 min. Subsequently, the SPME fiber (10 mm length × 50/30 μm thickness of DVB/CAR/PDMS film, Bellefonte, PA) was inserted into the headspace vial for 30 min. After absorption, the fiber was withdrawn into the needle holder, removed from the vial and introduced into a GC injector port for thermal desorption at 230 °C for 1 min with a splitless mode injection.GC-MS analysis
The GC-MS analysis was carried out on an Agilent gas chromatograph (HP 6890 N, Santa Clara, CA, USA) coupled to an Agilent MS detector (HP 5973 N, EI mode, Santa Clara, CA, USA). The separation was performed on a HP-5 MS capillary column (30 m × 0.32 mm i.d., 0.25 μm film thickness, Agilent Technologies, Santa Clara, CA, USA). The following parameters were applied: air flow, was set at 1.2 mL min−1 (He); the oven temperature was initialized at 40 °C for 5 min, followed by a 5 °C min−1 increase to 100 °C; finally reaching 230 °C with a heating rate of 20 °C min−1 and held for 3.5 min. The identification of volatile compounds was based on MS analysis by comparing the mass spectra data with those of authentic compounds available in the mass spectra library (NIST 02 mass spectra database). The approximate quantity of these volatile compounds from each sample was evaluated by the peak area ratio of their peak areas to that of the internal standard. This only allowed comparison of the relative contributions of the volatiles in the headspace of different model systems, and did not provide absolute concentrations in the aqueous solutions. Results were expressed as mean values of independent experiments (each experiment was triplicated).Browning intensity of Maillard reaction products
The browning intensity of Maillard reaction products was tested by colour development with a tristimulus reflectance colourmeter and measurement of absorbance reading at certain wavelength with a spectrophotometer. After GC analysis, the reaction mixtures were diluted (4×) with 50% aqueous methanol for the browning measurement. The diluted solution (4 mL) was transferred to Falcon centrifuge tubes and centrifuged (8000g) for 10 min. The L*, a*, b* values of the supernatant were measured with a Minolta Chorma meter (CR-400, Japan), where the three coordinates of the colour space represent the lightness of the colour (L* = 0 indicates black and L* = 100 indicates diffuse white), its position between red and green (a*, negative values indicate green while positive values indicate red) and its position between yellow and blue (b*, negative values indicate blue and positive values indicate yellow).22The reaction mixtures were also diluted to reasonable concentrations (absorbance range from 0.3 to 0.7) with 50% aqueous methanol before measuring the absorbance at 420 nm to evaluate the formation of brown polymers.23
LC-DAD-ESI/MS analysis of Maillard reaction products
The reaction mixtures were further analyzed on a HPLC-DAD-MS instrument equipped with an electrospray ionization (ESI) source interfaced to a QTRAP 4000 mass spectrometer (Applied Biosystems, CA, USA). Liquid chromatography was run on a separation model (Agilent 1200; Agilent Technologies, CA, USA) with a degasser, a quaternary pump, a thermostatted autosampler, and a photodiode array UV-vis detector. Separation of Maillard reaction products was conducted on an YMC-pack Pro column (5 μm, 2.1 × 150 mm). The mobile phase was composed of 0.1% formic acid water (solvent A) and acetonitrile (solvent B) of the following gradients: 0 min, 3% B; 5 min, 3% B; 35 min, 50% B; 45 min, 80% B; 55 min, 90%; returned to initial condition (3% B) in 1 min, and conditioned the column for 14 min. The total running time was 70 min for each sample. The flow rate was set at 0.2 mL min−1. The MS conditions were as follows: negative ion mode; curtain gas = 20; nebulizer gas flow = 40; heater gas flow = 40; spray voltage = 4.3 kV, scan range 200–1000 Da, DP (declustering potential) = 65 V. Enhanced mass spectrum (EMS) and enhanced product ion (EPI) scan model were used.Results and discussion
Effects of phloretin and phloridzin on the formation of Maillard reaction products in model systems with different reactant ratios of lysine and glucose
Several chemical model reactions were carried out with different ratios of lysine and glucose to investigate the effects of phloretin and phloretin on the formation of Maillard-type volatile products under mild thermal conditions (140 °C, 30 min). In total, 19 peaks were separated on a HP-5 MS capillary column, and were identified by comparing the mass spectra data with those of authentic compounds available in the Nist 02 mass spectra database. Quantification was on the basis of the adjusted peak area ratios of volatiles to the internal standard (Table 1). Firstly, it was discovered that with head-space solid phase micro-extraction (HS-SPME), the volatiles which can be identified from the reaction between lysine and glucose, were dominated by nitrogen-containing heterocyclic compounds, such as pyrazine and its derivatives, consistent with what is reported in the literature.24,25 The content of lysine was found to significantly affect the number and concentration of the volatile compounds formed. Furfural, 1,2,3,4-tetrahydroquinoline and eight pyrazines were identified in the model with a reactant ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (lysine
3 (lysine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose), while nine additional compounds such as 3-ethyl-2,5-dimethyl-pyrazine, N-ethyl-2,3-xylidine, 2,3-dihydro-1-methyl-1H-indole and 6-methyl-1,2,3,4-tetrahydroquinoline were only detected in models with reactant ratios of 1
glucose), while nine additional compounds such as 3-ethyl-2,5-dimethyl-pyrazine, N-ethyl-2,3-xylidine, 2,3-dihydro-1-methyl-1H-indole and 6-methyl-1,2,3,4-tetrahydroquinoline were only detected in models with reactant ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It was also found that the concentration of the major volatile compounds increased with increased amounts of lysine. For example, the amount of 2,5-dimethyl-pyrazine, the most dominant pyrazine found in the reaction mixtures at all reactant ratios in the model systems, showed a gradual increase from 3.53, 14.45 to 23.45 in models with reactant ratios of 1
1. It was also found that the concentration of the major volatile compounds increased with increased amounts of lysine. For example, the amount of 2,5-dimethyl-pyrazine, the most dominant pyrazine found in the reaction mixtures at all reactant ratios in the model systems, showed a gradual increase from 3.53, 14.45 to 23.45 in models with reactant ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Secondly, it was found that phloretin and phloridzin treatment has inhibitory effect on the formation of volatile compounds and most of the volatiles were significantly suppressed in samples treated with phloretin and phloridzin, with some even falling below the detection limit in model with reactant ratio of 1
1, respectively. Secondly, it was found that phloretin and phloridzin treatment has inhibitory effect on the formation of volatile compounds and most of the volatiles were significantly suppressed in samples treated with phloretin and phloridzin, with some even falling below the detection limit in model with reactant ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Meanwhile, phloretin was found to be more effective against the formation of Maillard-type volatiles than phloridzin. The similar inhibitory effect of phenolic compounds on the formation of Maillard-type volatile compounds was reported in previous studies,17,26 such as epicatechin, epicatechin gallate and epigallocatechin gallate, which could significantly reduce the formation of pyrazine and its derivatives. Considering that MGO and GO were the main precursors for the formation of pyrazines,24 and that phloretin and phloridzin have been found to be effective MGO and GO scavengers under physiological conditions.20 It is reasonable to assume that the inhibitory effects of phloretin and phloridzin on the formation of Maillard-type volatiles were attributed to their quenching ability with reactive carbonyl species. Meanwhile, the data also showed that lower inhibitory efficiencies of phloretin and phloridzin on the formation of volatile compounds appeared in models with a higher lysine content, suggesting the competition of phloretin/phloridzin and amino groups for the reactive carbonyl species (such as MGO and GO).
3. Meanwhile, phloretin was found to be more effective against the formation of Maillard-type volatiles than phloridzin. The similar inhibitory effect of phenolic compounds on the formation of Maillard-type volatile compounds was reported in previous studies,17,26 such as epicatechin, epicatechin gallate and epigallocatechin gallate, which could significantly reduce the formation of pyrazine and its derivatives. Considering that MGO and GO were the main precursors for the formation of pyrazines,24 and that phloretin and phloridzin have been found to be effective MGO and GO scavengers under physiological conditions.20 It is reasonable to assume that the inhibitory effects of phloretin and phloridzin on the formation of Maillard-type volatiles were attributed to their quenching ability with reactive carbonyl species. Meanwhile, the data also showed that lower inhibitory efficiencies of phloretin and phloridzin on the formation of volatile compounds appeared in models with a higher lysine content, suggesting the competition of phloretin/phloridzin and amino groups for the reactive carbonyl species (such as MGO and GO).
| Compound | Lys![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glu = 1 Glu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Lys![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glu = 1 Glu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Lys![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glu = 2 Glu = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Pz-treated | Ph-treated | Control | Pz-treated | Ph-treated | Control | Pz-treated | Ph-treated | |
| a Approximate quantities in the headspace given as the means of independent experiments (peak area ratio of volatile compounds to internal standard (n-dodecane)). b Each model system consisted of different reactant ratios in 1.0 mL phosphated buffer (0.1 M, pH 7.4). Lys = lysine; Glu = glucose. c Pz-treated: model systems treated with phloridzin; Ph-treated: model systems treated with phloretin. | |||||||||
| Pyrazine | 2.40 | 2.32 | 0.55 | 3.23 | 2.82 | 2.18 | 3.51 | 3.04 | 2.82 |
| Methyl-pyrazine | 3.64 | 2.64 | 1.55 | 7.91 | 7.45 | 3.27 | 10.82 | 8.36 | 7.05 |
| Furfural | 0.45 | ||||||||
| 2,5-Dimethyl-pyrazine | 3.53 | 1.69 | 0.49 | 14.45 | 11.55 | 5.45 | 23.45 | 23.18 | 14.73 |
| 2,3-Dimethyl-pyrazine | 1.25 | 4.55 | 1.82 | 0.91 | 6.73 | 3.73 | 2.51 | ||
| 2-Ethyl-6-methyl-pyrazine | 0.23 | 1.23 | 0.78 | 0.55 | 2.73 | 2.56 | 1.32 | ||
| 2-Ethyl-5-methyl-pyrazine | 0.55 | 3.73 | 2.64 | 1.82 | 7.27 | 6.36 | 4.55 | ||
| Trimethyl-pyrazine | 0.73 | 5.82 | 2.91 | 1.91 | 14.09 | 8.36 | 7.23 | ||
| (1-Methylethenyl)-pyrazine | 0.38 | 1.91 | 1.27 | 2.68 | 2.25 | 0.73 | |||
| 3-Ethyl-2,5-dimethyl-pyrazine | 1.55 | 1.00 | 0.32 | 5.55 | 5.32 | 4.68 | |||
| 2-Methyl-5-(2-propenyl)-pyrazine | 2.08 | 1.00 | 4.91 | 3.59 | 1.64 | ||||
| 5,6,7,8-Tretrahydroindolizine | 2.77 | 2.59 | 1.13 | 9.27 | 3.41 | 2.18 | |||
| N,4-Dimethyl-benzenamine | 1.80 | 1.45 | 1.91 | 5.73 | 2.29 | 1.09 | |||
| 2,3-Dihydro-1-methyl-1H-indole | 3.68 | 1.59 | 0.45 | 7.82 | 3.09 | 1.98 | |||
| 2,4,6-Trimethyl-benzenamine | 10.18 | 10.73 | 1.50 | 29.09 | 17.45 | 8.36 | |||
| 1,2,3,4-Tetrahydroquinoline | 3.33 | 1.14 | 11.05 | 4.55 | 0.50 | 12.55 | 9.64 | 7.45 | |
| N-Ethyl-2,3-xylidine | 1.18 | 0.55 | 4.09 | 1.86 | 0.95 | ||||
| 2,3-Cycloheptenopyridine | 4.00 | 0.23 | 4.36 | 1.09 | 0.62 | ||||
| 6-Methyl-1,2,3,4-tetrahydroquinoline | 3.18 | 0.69 | 3.82 | 1.77 | 1.41 | ||||
It is well-known that the colour formed in food processing is one of the most important parameters that affects customer perceptions of food. Meanwhile, the colour formation is closely related to the Maillard reaction in thermal processing. Therefore, an experiment was carried out to evaluate the capacity of phloretin and phloridzin to impact the colour development in the lysine–glucose model with different reactant ratios. It was found that with heating the model systems for 30 min, the formation of a brown colour was observed and could be monitored with a wavelength of 420 nm. It was also found that the browning formation was enhanced with increased contents of lysine in the three individual models, which confirmed that the concentration of amino group directly determines the rate of the Maillard reaction. No browning colour was observed in the samples only containing phloretin and/or phloridzin, subsequent HPLC analysis showed that the phloretin and/or phloridzin did not change. Therefore, phloretin and/or phloridzin, when heated alone, could not contribute to colour changes in the lysine–glucose model systems treated with phloretin and/or phloridzin. Moreover, it was found that with the same reactant ratios, the phloretin-treated system showed the highest absorbance, while a similar absorbance was observed in both the phloridzin-treated and corresponding control systems, which suggests that phloretin could actively participate in the Maillard reaction and promote the formation of coloured compounds in 30 min, while phloridzin showed weaker effects.
Apart from the spectrophotometric measurement, colourimetric analysis was also applied to investigate the effect of the addition of phloretin and phloridzin on all of the three coordinates of colour space, as reflected by changes in L*, a*, and b* values in the lysine–glucose model systems. As shown in Fig. 1, phloretin and phloridzin could impact the colour development in the lysine–glucose model systems, and the effect was dependent on the reactant ratios of the model systems. In terms of lightness (L*) (Fig. 1A), the phloretin-treated samples were lower than the other two model samples at the same reactant ratio. As the reactant ratio increased, lightness (L*) of the reaction systems decreased gradually but with different rates in the different treatment models. In particular, the most drastic decrease in lightness was observed in the phloretin-treated model. Precipitate was observed in the phloretin-treated model system, and the amount increased with the amount of lysine, which suggested that phloretin would probably be involved in the condensation reactions with amino acids and sugars to form phenol resins.27 In terms of redness (a*), interestingly, the control and phloridzin-treated samples increased as the content of lysine increased, but decreased in the phloretin-treated samples (see Fig. 1B). Sawhney et al.28 found that some oxidated products of phenols were formed in the presence of air during the heating, which could induce the red-shift of the absorption band. In this study, phloretin might easily transform into its quinone structure during heating, and therefore is accompanied with high redness (positive a* values) at low ratios of lysine to glucose. In the model with high concentrations of lysine, the high rate of the Maillard reaction might compel the phloretin and its quinone to undergo further reactions, such as polymerization, resulting in a decrease in redness. Overall, phloretin and phloridzin could significantly affect the colour development in the lysine–glucose model systems.
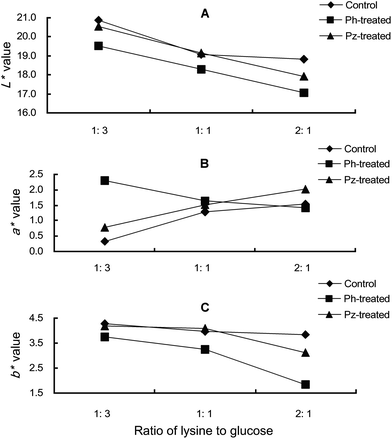 | ||
Fig. 1 The readings of L*, a* and b* values in the lysine–glucose model systems with or without phloretin and phloridzin treatments. A, B and C: readings of L*, a* and b* values, respectively; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1 3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2 1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1: model systems with reactant ratios (lysine 1: model systems with reactant ratios (lysine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose) of 1 glucose) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1 3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2 1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. 1, respectively. | ||
Given the results of both the spectrophotometric measurement and colorimetric analysis, the addition of phloretin and phloridzin could affect the colour development of Maillard reaction products in the lysine–glucose model. Phloretin could significantly promote the brown colour formation. Meanwhile, phloretin and phloridzin might be directly involved in the Maillard reaction.
These experimental results clearly demonstrate that phloretin and phloridzin could significantly impact the formation of Maillard reaction products. To further investigate how phloretin and phloridzin affect the formation of Maillard reaction products in the lysine–glucose model, the reaction mixtures were diluted 100-fold with 50% aqueous methanol, then underwent syringe-driven filtering and were then subjected to HPLC and LC-DAD-ESI/MS analysis. For the phloretin-treated model systems, it was found that after a 30 min reaction time, no apparent peak [HPLC chromatogram (284 nm) not shown] was observed for phloretin, which means that the phloretin was fully consumed within the Maillard reaction in the lysine–glucose model. Meanwhile, there were no additional peaks with similar UV characteristics to the phloretin observed in this HPLC chromatogram, suggesting that phloretin could be involved in a series of complicated reactions. In the phloridzin-treated model systems, the amount of phloridzin decreased gradually with increased amounts of lysine. Small amounts (about 0.07 mM) of phloretin was also detected in one model system (lysine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose, 1
glucose, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), which indicated that deglycosylation of phloridzin had occurred under the thermal processing. Moreover, there are three additional peaks appearing between the peaks of phloridzin and phloretin. These additional peaks showed similar UV characteristics to phloridzin and phloretin. It is reasonable to assume that these peaks are adducts associated with phloretin and phloridzin. Given the higher reactivity of phloretin than that of phloridzin, the heating time was shortened to 7 min for the model systems containing phloretin, in order to investigate the possible reactions that happen to phloretin in the initial steps. It was found that in these modified model systems, the concentration of phloretin gradually decreased until it was completely consumed. At the reactant ratio of 1
3), which indicated that deglycosylation of phloridzin had occurred under the thermal processing. Moreover, there are three additional peaks appearing between the peaks of phloridzin and phloretin. These additional peaks showed similar UV characteristics to phloridzin and phloretin. It is reasonable to assume that these peaks are adducts associated with phloretin and phloridzin. Given the higher reactivity of phloretin than that of phloridzin, the heating time was shortened to 7 min for the model systems containing phloretin, in order to investigate the possible reactions that happen to phloretin in the initial steps. It was found that in these modified model systems, the concentration of phloretin gradually decreased until it was completely consumed. At the reactant ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, seven additional peaks appeared and showed similar UV absorption to that of phloretin. Moreover, the number of those peaks decreased in the model systems with the two other reactant ratios, indicating that these phloretin adducts potentially could be involved in further complicated reactions to form more complex products that can not be detected by our current HPLC method.
3, seven additional peaks appeared and showed similar UV absorption to that of phloretin. Moreover, the number of those peaks decreased in the model systems with the two other reactant ratios, indicating that these phloretin adducts potentially could be involved in further complicated reactions to form more complex products that can not be detected by our current HPLC method.
Apart from the HPLC-DAD analysis, the MS data of the reaction mixtures was obtained simultaneously. The LC-MS TIC (total ion chromatograms) of these model systems (model samples with phloretin treatment were heated only for 7 min, while others were heated for 30 min) was shown in Fig. 2 with the adduct peaks marked with numbers, I, II, III, IV, V, VI, VII, VIII, IX and X, based on their elution order. Their predicted molecular weight was suggested as 436, 434, 436, 418, 508, 418, 492, 346, 344 and 316 (m/z 435, 433, 435, 417, 507, 417, 491, 345, 343 and 315), respectively (Fig. 3A). It has been reported by other research groups that polyphenols, such as epicatechin and other flavan-3-ols, could directly trap sugar fragments in low-moisture and aqueous Maillard model systems,17–19,26 and phloretin can directly trap reactive dicarbonyl species, such as methylglyoxal, under physiological conditions.20 Therefore, these analytes may be adducts formed from phloretin or phloridzin with the key sugar fragments generated from the Maillard reaction. These analytes were further identified with EPI (MS/MS technique) and their EPI spectrum had a typical loss of 106 amu (the B ring unit of phloretin or phloridzin), which is similar to that of mono-MGO-phloretin and di-MGO-phloretin adducts.20 Moreover, the neutral loss of 162 amu (glucose residue loss) could be easily observed in the EPI spectra of the analytes with m/z of 507 and 491, which indicated that these two adducts were phloridzin-associated products. To further validate this hypothesis, these possible phloretin or phloridzin-sugar fragment adducts was identified with a model reactions using a CAMOLA technique29 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 unlabelled/labelled glucose) viaHPLC-MS analysis. In the CAMOLA reaction, the thermally induced breakdown of the carbohydrate skeleton, followed by recombination of the intermediates formed, generates a mixture of isotopomers of the target molecule from which the importance of single reaction pathways can be deduced based on mass spectroscopic data and statistical rules. In the current study, briefly, the mass shift of the analyte, due to heavy isotope incorporation and statistical distribution of isotopomers, would help to figure out the type of sugar fragments that are combined with phloretin and phloridzin. The mass spectra of the potential phloretin and phloridzin-sugar fragmentation products formed in CAMOLA reaction was illustrated in Fig. 3B. It was observed that phloridzin adducts with m/z [(M − H)−] of 507 and 491 showed equal abundance to their isotopomers of 510 and 494 (both have a 3 amu mass shift) in the phloridzin-treated model system with the CAMOLA technique, which indicated that the sugar fragments incorporated with phloridzin contained three carbon atoms, and these correspond to C3H4O2 and C3H4O fragments, respectively. Similarly, analytes with m/z 435, 433, 435, 417, 417, 345, 343 and 315 were assigned to C6 (C6H10O5), C6 (C6H8O5), C6 (C6H10O5), C6 (C6H8O4), C6 (C6H8O4), C3 (C3H4O2), C3 (C3H2O2) and C2 (C2H2O) with phloretin, respectively. Comparison of the adducts formed in the phloretin and phloridzin-treatment model systems showed that phloretin can directly react with glucose and its dehydrated products (intact C6sugar fragments) formed from the initial steps of the Maillard reaction, while phloridzin can only react with sugar fragments (reactive carbonyl species, mainly intact C3 sugar fragments) formed in the advanced steps of the Maillard reaction, suggesting that phloretin has a higher reactivity than phloridzin in the Maillard reaction systems.
1 unlabelled/labelled glucose) viaHPLC-MS analysis. In the CAMOLA reaction, the thermally induced breakdown of the carbohydrate skeleton, followed by recombination of the intermediates formed, generates a mixture of isotopomers of the target molecule from which the importance of single reaction pathways can be deduced based on mass spectroscopic data and statistical rules. In the current study, briefly, the mass shift of the analyte, due to heavy isotope incorporation and statistical distribution of isotopomers, would help to figure out the type of sugar fragments that are combined with phloretin and phloridzin. The mass spectra of the potential phloretin and phloridzin-sugar fragmentation products formed in CAMOLA reaction was illustrated in Fig. 3B. It was observed that phloridzin adducts with m/z [(M − H)−] of 507 and 491 showed equal abundance to their isotopomers of 510 and 494 (both have a 3 amu mass shift) in the phloridzin-treated model system with the CAMOLA technique, which indicated that the sugar fragments incorporated with phloridzin contained three carbon atoms, and these correspond to C3H4O2 and C3H4O fragments, respectively. Similarly, analytes with m/z 435, 433, 435, 417, 417, 345, 343 and 315 were assigned to C6 (C6H10O5), C6 (C6H8O5), C6 (C6H10O5), C6 (C6H8O4), C6 (C6H8O4), C3 (C3H4O2), C3 (C3H2O2) and C2 (C2H2O) with phloretin, respectively. Comparison of the adducts formed in the phloretin and phloridzin-treatment model systems showed that phloretin can directly react with glucose and its dehydrated products (intact C6sugar fragments) formed from the initial steps of the Maillard reaction, while phloridzin can only react with sugar fragments (reactive carbonyl species, mainly intact C3 sugar fragments) formed in the advanced steps of the Maillard reaction, suggesting that phloretin has a higher reactivity than phloridzin in the Maillard reaction systems.
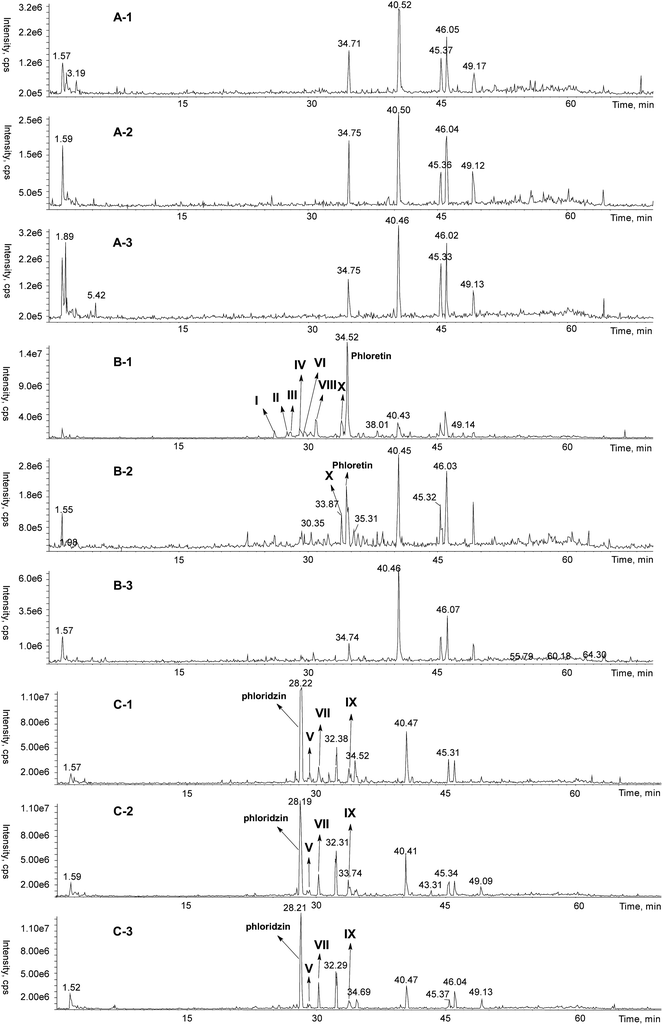 | ||
Fig. 2 Typical HPLC-MS chromatograms of the different Maillard model systems. (A) Control samples with different reactant ratios, (B) samples treated with phloretin (heated for 7.0 min) and (C) samples treated with phloridzin. −1, −2 and −3 for model systems with reactant ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1 3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2 1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. I, II, III, IV, V, VI, VII, VIII, IX and X were used to label the potential adducts associated with phloretin or phloridzin. 1, respectively. I, II, III, IV, V, VI, VII, VIII, IX and X were used to label the potential adducts associated with phloretin or phloridzin. | ||
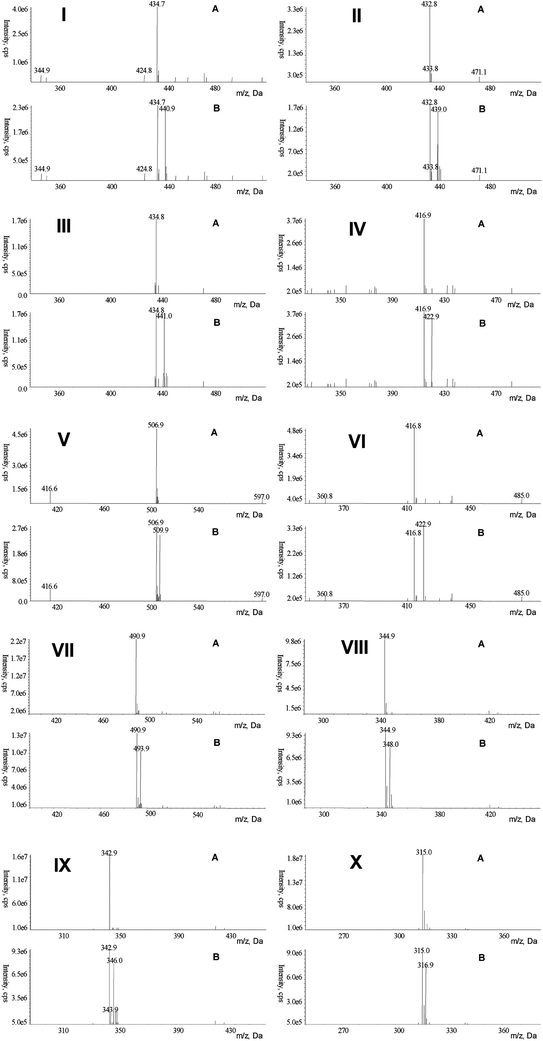 | ||
| Fig. 3 Mass spectra of potential phloretin/phloridzin adducts formed in the normal and CAMOLA reaction mixtures. Compounds I, II, III, IV, V, VI, VII, VIII, IX and X were consistent with the marked numbers in Fig. 2. (A) Normal reaction mixture; (B) CAMOLA reaction mixture. | ||
Effects of phloretin and phloridzin on the formation of Maillard reaction products in the AC–glu and AG–glu model systems
As in real food systems, the reactive amino groups for the Maillard reaction mainly come from peptides and proteins where α-amino groups are substituted by peptide bonds, instead of free amino acids.30 It is necessary to block the α-amino group of lysine to mimic the Maillard reaction in real food systems and to study the effects of phloretin and phloridzin on these reactions. Therefore, AC–glu and AG–glu model systems were employed to investigate the effects of phloretin and phloridzin on the formation of Maillard reaction products in the current study. Similarly, the formation of volatile compounds and colour development were applied as two key parameters to evaluate the capacity of phloretin and phloridzin on these model systems. GC-MS was applied to identify the volatiles formed. As a result, the most abundant volatile compounds detected in the AC–glu or AG–glu model systems with or without the addition of phloretin or phloridzin are listed in Table 2. In comparison with the lysine–glucose model system, the volatile compounds formed in the AC–glu and AG–glu model systems changed dramatically. The abundant volatile products detected in the AC–glu and AG–glu model systems were 2,3-butanedione, toluene, furfural and 2,5-furandicarboxaldehyde. Pyrazine and its derivatives, the dominant products formed in the lysine–glucose model systems, were not detected. This phenomenon would be likely due to the strong reactivity of the α-amino group of lysine, making it easy to dissociate from the lysine skeleton via the Strecker degradation reaction and to form aminoketones, thus leading to the generation of pyrazine and its derivatives.25,31 Although the volatile compounds formed are different in these model systems, the addition of phloretin and phloridzin still significantly inhibits their formation. In these systems, the treatment with phloretin led the concentration of 2,3-butanedione, 2,5-furandicarboxaldehyde to fall below the detection limit of GC-MS. The brown intensity of these reaction mixtures was further monitored with spectrophotometric measurement at 420 nm and the results are shown in Fig. 4. It is indicated that phloretin and phloridzin could also promote the colour development in these Maillard reactions. Phloretin could significantly promote the formation of brown compounds in both the AC–glu and AG–glu model systems. Their values were two times higher than that in the corresponding control samples. However, the highest brown intensity was observed in the AC–glu and AG–glu model systems, which was only one-fifth or one-ninth of that observed in the lysine–glucose model systems. Further HPLC-DAD-ESI/MS analysis of the diluted (100-fold) reaction solution indicated that phloretin and phloridzin could directly trap the reactive sugar fragments in both the Ac–glu and AG–glu model systems (data not shown). Similar phloretin and phloridzin adducts were produced in both the AC–glu and AG–glu model systems. However, in these two systems, high amounts of phloretin and phloridzin still remained in the reaction mixture, which indicted that sugar fragments formation rate was lower in the AC–glu and AG–glu model systems than in the lysine–glucose model systems.| Compound | AC–glu | AG–glu | ||||
|---|---|---|---|---|---|---|
| Control | Pz-treated | Ph-treated | Control | Pz-treated | Ph-treated | |
| a Approximate quantities in the headspace are given as the means of independent experiments (peak area ratios of volatile compounds to the internal standard (n-dodecane)). b AC–glu: Nα-acetyllysine–glucose model system; AG–glu: N-acetyl-gly-lys methyl ester acetate salt–glucose model system. c Pz-treated: model systems treated with phloridzin; Ph-treated: model systems treated with phloretin. | ||||||
| 2,3-Butanedione | 3.00 | 1.55 | 2.64 | 1.36 | ||
| Toluene | 0.82 | 0.50 | 0.58 | 0.69 | 0.44 | 0.43 |
| Furfural | 0.73 | 0.19 | 0.09 | 0.53 | 0.18 | 0.06 |
| 2,5-Furandicarboxaldehyde | 1.24 | 0.44 | 2.14 | 0.38 | ||
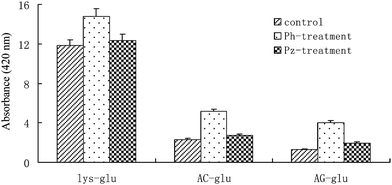 | ||
Fig. 4 Absorbance value at 420 nm in model systems with and without treatment with phloretin and/or phloridzin. The ratios of L-lys, AC-lys and AG-lys to glucose was all 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the model systems. Corrected absorbance: (A) × dilution values; results are means of three replicates. 1 in the model systems. Corrected absorbance: (A) × dilution values; results are means of three replicates. | ||
Conclusion
In summary, this study demonstrates the active roles of phloretin and phloridzin in the formation of Maillard reaction products in model systems composed of L-lysine–glucose and L-lysine derivates–glucose under aqueous condition. Overall, phloretin and phloridzin could inhibit the formation of Maillard-type volatile products and significantly affect the colour development in the above model systems. With the CAMOLA technique and HPLC-DAD-ESI/MS analysis, it was tentatively demonstrated that the capability of phloretin and phloridzin to trap reactive sugar fragments, key intermediates of the Maillard reaction, might be responsible for their effects on flavours and colour formation. Ultimately, a better understanding of the influence of phenolic compounds on Maillard reactions may provide an alternative way to influence the flavour properties, colour formation and nutritional value, and therefore improve the quality of food products.Acknowledgements
The equipment used in this work were partially supported by the Special Equipment Grant from the University Grants Committee of the Hong Kong Special Administrative Region, China (Project Code: SEG_HKU02).References
- E. Bailey Milton, in The Maillard Reaction in Foods and Nutrition, American Chemical Society, 1983, vol. 215, pp. 169–184 Search PubMed.
- H.-T. Cheng, W.-F. Lin and C.-R. Wang, in The Maillard Reaction in Foods and Nutrition, American Chemical Society, 1983, vol. 215, pp. 91–102 Search PubMed.
- C. C. Tsen, K. P. R. Reddy, K. S. El-Samahy and W. C. Gehrke, in The Maillard Reaction in Foods and Nutrition, American Chemical Society, 1983, vol. 215, pp. 379–394 Search PubMed.
- M. C. Nicoli, M. Anese, L. Manzocco and C. R. Lerici, LWT–Food Sci. Technol., 1997, 30, 292–297 Search PubMed.
- C. M. Brands, G. M. Alink, M. A. van Boekel and W. M. Jongen, J. Agric. Food Chem., 2000, 48, 2271–2275 CrossRef CAS.
- T. Shinoda, F. Hayase, N. Vanchuyen and H. Kato, Biosci., Biotechnol., Biochem., 1993, 57, 1826–1831 Search PubMed.
- C. Thongraung and S. Kangsanan, Int. J. Food Sci. Technol., 2010, 45, 1696–1702 Search PubMed.
- C. P. Sherwin and T. P. Labuza, J. Food Sci., 2003, 68, 588–594 Search PubMed.
- F. J. Moreno, E. Molina, A. Olano and R. Lopez-Fandino, J. Agric. Food Chem., 2003, 51, 394–400 CrossRef CAS.
- T. Hofmann and P. Schieberle, J. Agric. Food Chem., 1995, 43, 2187–2194 Search PubMed.
- H. G. Xu, X. Liu, J. Zhao and Y. X. Gao, Food Res. Int., 2008, 41, 730–737 Search PubMed.
- M. L. Wolfrom, N. Kashimur and D. Horton, J. Agric. Food Chem., 1974, 22, 796–800 Search PubMed.
- B. L. Wedzicha and M. T. Kaputo, Food Chem., 1992, 43, 359–367 Search PubMed.
- Y. Fujiwara, N. Kiyota, K. Tsurushima, M. Yoshitomi, K. Mera, N. Sakashita, M. Takeya, T. Ikeda, T. Araki, T. Nohara and R. Nagai, Free Radical Biol. Med., 2011, 50, 883–891 Search PubMed.
- P. M. Colahan-Sederstrom and D. G. Peterson, J. Agric. Food Chem., 2005, 53, 398–402 Search PubMed.
- S. L. Schwambach and D. G. Peterson, J. Agric. Food Chem., 2006, 54, 502–508 Search PubMed.
- V. M. Totlani and D. G. Peterson, J. Agric. Food Chem., 2005, 53, 4130–4135 Search PubMed.
- V. M. Totlani and D. G. Peterson, J. Agric. Food Chem., 2006, 54, 7311–7318 CrossRef CAS.
- V. M. Totlani and D. G. Peterson, J. Agric. Food Chem., 2007, 55, 414–420 Search PubMed.
- X. Shao, N. Bai, K. He, C.-T. Ho, C. S. Yang and S. Sang, Chem. Res. Toxicol., 2008, 21, 2042–2050 CrossRef CAS.
- E. Ibañez and R. A. Bernhard, J. Sci. Food Agric., 1996, 72, 91–96 CrossRef CAS.
- H. Özoglu and A. BayIndIrlI, Food Control, 2002, 13, 213–221 Search PubMed.
- E. H. Ajandouz, L. S. Tchiakpe, F. D. Ore, A. Benajiba and A. Puigserver, J. Food Sci., 2001, 66, 926–931 Search PubMed.
- F. Van Lancker, A. Adams and N. De Kimpe, J. Agric. Food Chem., 2010, 58, 2470–2478 Search PubMed.
- H. I. Hwang, T. G. Hartman, R. T. Rosen, J. Lech and C. T. Ho, J. Agric. Food Chem., 1994, 42, 1000–1004 Search PubMed.
- Y. Noda and D. G. Peterson, J. Agric. Food Chem., 2007, 55, 3686–3691 Search PubMed.
- S. A. Sojka, R. A. Wolfe and G. D. Guenther, Macromolecules, 1981, 14, 1539–1543 Search PubMed.
- B. L. Sawhney, Clays Clay Miner., 1985, 33, 123–127 Search PubMed.
- P. Schieberle, Ann. N. Y. Acad. Sci., 2005, 1043, 236–248 Search PubMed.
- A. Arnoldi, in Nutrition Handbook for Food Processors, ed. C. J. K. Henry and C. Chapman, Woodhead Publishing, U. K., 2002, pp. 265–292 Search PubMed.
- H.-I. Hwang, T. G. Hartman and C.-T. Ho, J. Agric. Food Chem., 1995, 43, 179–184 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |
