Atmospheric deposition and storm induced runoff of heavy metals from different impermeable urban surfaces
Daniel
Wicke
*,
Thomas A.
Cochrane
and
Aisling D.
O'Sullivan
Hydrological and Ecological Engineering Research Group, Department of Civil and Natural Resources Engineering, University of Canterbury, Private Bag 4800, Christchurch, 8140, New Zealand. E-mail: daniel.wicke@gmx.de; Fax: +64 3 364 2758; Tel: +64 3 364 2378
First published on 14th November 2011
Abstract
Contaminants deposited on impermeable surfaces migrate to stormwater following rainfall events, but accurately quantifying their spatial and temporal yields useful for mitigation purposes is challenging. To overcome limitations in current sampling methods, a system was developed for rapid quantification of contaminant build-up and wash-off dynamics from different impervious surfaces. Thin boards constructed of concrete and two types of asphalt were deployed at different locations of a large carpark to capture spatially distributed contaminants from dry atmospheric deposition over specified periods of time. Following experimental exposure time, the boards were then placed under a rainfall simulator in the laboratory to generate contaminant runoff under controlled conditions. Single parameter effects including surface roughness and material composition, number of antecedent dry days, rain intensity, and water quality on contaminant build-up and wash-off yields could be investigated. The method was applied to quantify spatial differences in deposition rates of contaminants (TSS, zinc, copper and lead) at two locations varying in their distance to vehicle traffic. Results showed that boards exposed at an unused part of the carpark >50 m from vehicular traffic captured similar amounts of contaminants compared with boards that were exposed directly adjacent to the access route, indicating substantial atmospheric contaminant transport. Furthermore, differences in contaminant accumulation as a function of surface composition were observed. Runoff from asphalt boards yielded higher zinc loads compared with concrete surfaces, whereas runoff from concrete surfaces resulted in higher TSS concentrations attributed to its smoother surfaces. The application of this method enables relationships between individual contaminant behaviour and specific catchment characteristics to be investigated and provides a technique to derive site-specific build-up and wash-off functions required for modelling contaminant loads from impermeable surfaces.
Environmental impactHeavy metals in urban stormwater runoff remain a cause for concern and warrant ongoing research due to their persistence in the environment and adverse impact on receiving waterways. While vehicular traffic is recognised as a major source of these contaminants, their atmospheric transport and the effect of different impervious surfaces on contaminant behaviour is poorly understood. Nonetheless, quantifying these processes is necessary for accurately modelling contaminant distribution which guides Best Management Practices for stormwater mitigation. This paper describes the application of a new method that allows spatial quantification of contaminants from atmospheric deposition on different urban surfaces. Results indicate that atmospheric transport over distances >50 m occurs and that different surface material characteristics greatly influence runoff signatures. |
Introduction
Stormwater runoff from impervious surfaces in urban catchments (e.g. roads, carparks, roofs) is known to be a significant source of heavy metals and other potentially toxic compounds (e.g. as reviewed by Gobel et al.1) transporting non-point source pollution to neighbouring waterways. Although new emerging contaminants such as phthalates (used as additives in plastics, especially in PVC) have recently come into focus,2 heavy metals, particularly zinc (Zn), copper (Cu) and lead (Pb) continue to dominate stormwater signatures. For instance, Hjortenkrans et al.3 found that levels of copper and zinc emissions from brake linings and tire wear, respectively, were the same in 2005 compared to 1995/98, with an estimated load of 3,800 kg·yr−1 for copper and 5,200 kg·yr−1 for zinc in the city of Stockholm. Similarly, in a recent study in Australia, annual stormwater discharge to the Sydney estuary was estimated to be as high as 3,200 kg·yr−1 for copper, 17,700 kg·yr−1 for zinc and 3,600 kg·yr−1 for lead.4 Various studies have identified the traffic sector as a main source of these metals, e.g. by quantifying metal emissions in road tunnels,5 analyzing urban metal flows6 or by finding strong correlations between heavy metals in bulk atmospheric deposition and road proximity and traffic volume in the Sydney Metropolitan Area.7 Besides traffic, building materials such as galvanized iron and copper roofs are other significant sources of copper and zinc in stormwater.8,9 Due to its toxicity, untreated storm runoff negatively impacts aquatic ecosystems in receiving waterways that typically serve as stormwater drainage systems.10–12Most stormwater studies focus on quantifying concentrations and yields of the key pollutants (e.g.1, 13), investigating the ecotoxicity of runoff (e.g.10, 12), modelling pollution build-up and contaminated flow pathways (e.g.14), or determining efficiencies of various treatment approaches (e.g.15). However, there is a dearth of information regarding the contribution of atmospheric-driven contaminant transport and deposition on impervious surfaces and resulting contaminant spatial variability in stormwater runoff. Sabin et al.16 reported that atmospheric deposition accounted for >50% of total heavy metal loads in stormwater within the semi-arid study area in Los Angeles. They also monitored particulate metals originating from vehicular traffic at a distance of 10–450 m downwind of a busy freeway but did not examine specific build-up or wash-off relationships for different catchment conditions.17 Davis and Birch7 investigated spatial distribution of atmospheric deposition of heavy metals within an urban catchment (suburb of Sydney metropolitan area) and quantified pollutant fluxes beside roads of different traffic intensity, but did not take deposition and wash-off characteristics from urban impervious surfaces into account as atmospheric deposition was collected in plastic tanks. Quantifying spatial variability in contaminant loads as a function of different pavement types is needed to help guide Best Management Practices (BMPs) for stormwater mitigation.
Various manual and automatic sampling methods are currently employed for collecting contaminated runoff from impervious surfaces. Direct runoff sampling as grab or composite samples during event-driven precipitation events,10,18,19 employing rainfall simulators to wash off contaminants at the field site,11,20,21 and collecting runoff from roof materials in the laboratory under controlled conditions9 are all reported. Although direct runoff sampling during a storm event can estimate contaminant contributions, it is often logistically challenging, expensive and time-consuming to conduct accurately and frequently, and is often conducted at discharge points within the catchment which does not provide much information on the spatial contaminant distribution patterns. Furthermore, inherent variability in natural rainfall event conditions such as the number of antecedent dry days, rainfall intensity, and storm duration makes it difficult to investigate the effect of single parameters on contaminant yields limiting the opportunity to derive contaminant build-up and wash-off functions required for robust stormwater modelling. The use of rainfall simulators in the field could overcome some of these limitations, but transporting a relatively large rainfall simulator and associated components (e.g. feed water tank, piping, monitoring instrumentation, power) to a field research site is logistically challenging and time-consuming if many sites are to be investigated to obtain spatially-distributed contaminant runoff.
To overcome these limitations we developed an easily deployable experimental method for capturing contaminants on different impervious surfaces to investigate individual parameter effects on contaminants, delineate their spatial variability, and develop build-up and wash-off functions for different surface types necessary for stormwater contaminant models. In this paper we present results from the application of this method to understand and quantify differences in TSS and heavy metal dry deposition at the entrance of a large carpark and in an unused part of that carpark over 50 meters away from any traffic. We relate build-up and wash-off rates to the roughness and composition characteristics of asphalt and concrete impermeable surfaces that may be found at these locations.
Materials and methods
Construction of impervious boards
Asphalt and concrete boards (75 cm L × 75 cm W × 3 cm D; 0.56 m2) were designed to capture key contaminants (metals and solids) accumulating over time in an urban catchment. Twelve boards of three different types (four replicates) were constructed to provide different surface roughness and composition of the urban paving materials: concrete, smooth (3 mm max.) and coarse (14 mm max.) aggregate asphalt. Concrete mixture was prepared from 4.2 kg cement, 2.7 L tap water, 15 kg of 5 mm gravel, 13.5 kg sand, 50 g shogun (plastic) fibers, 15 g sika (polymer) megafibers, and 13 mL water reducer (Plastiment RMC01, Sika Ltd.) for each board. Asphalt boards were constructed at a commercial road construction site by Fulton Hogan Construction (Christchurch, New Zealand) using either the 3 mm or 14 mm maximum aggregate size asphalt. All asphalt boards were compacted using a standard plate compactor to achieve asphalt characteristics analogous to local roadways.Surface roughness
The constructed boards were assessed for differences in surface roughness using a surface laser scanner as described in Darboux et al.22 Resulting elevation matrices were analyzed in ArcGIS 9.3 (ESRI, USA) to generate three-dimensional images to visualize the surface topography (Fig. 1), where darker shades represent lower elevation matrices indicating deeper pits. Surface roughness was estimated by determining the minimum and maximum elevations in relation to the mean planar layer across each board type (concrete: min. −0.6 mm, max. 0.6 mm; smooth asphalt: min. −2.4 mm, max. 3.2 mm; coarse asphalt: min. −3.2 mm, max. 5.9 mm). Standard deviations in surface elevation derived from the elevation matrices quantifying surface roughness characteristics of each board type were 0.13 mm for concrete, 0.51 mm for smooth asphalt (3 mm maximum aggregate) and 0.76 mm for coarse asphalt (14 mm maximum aggregate). The surface characteristics of the smooth asphalt boards resembled the asphalt used in the actual car park location of the experiments.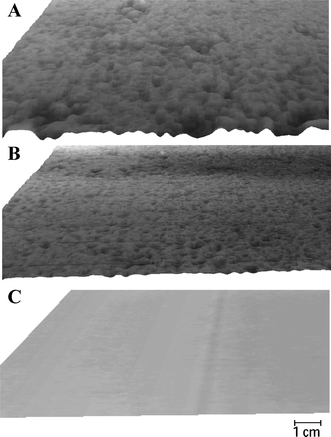 | ||
| Fig. 1 Three-dimensional images of different board types as derived from surface laser scanner. A – coarse (14 mm) asphalt, B – smooth (3 mm) asphalt, C – concrete. Heights are represented by differences in grey levels with dark tones showing lower elevations. | ||
Exposure of boards to determine contaminant accumulation
Contaminants for each impervious surface type were collected by deploying the boards in the main University of Canterbury carpark (1.6 ha, 650 lots). One set of six boards, consisting of two replicates of each surface type, was placed for 7 dry days directly beside the curb of the main carpark entrance and exit (cars could not drive over boards). It was expected that this location would have higher contaminant depositions due to the proximity (∼1 m) to vehicle traffic. The number of cars passing the boards during time of exposure was determined using a two-tube portable vehicle classifier (MetroCount MC5600). A second set of six boards was placed at the same time in a blocked-off parking lot in an unused area of the carpark at a distance of >50 m from the nearest used parking lot (Fig. 2c). This area was expected to represent contaminants accumulating from atmospheric deposition not directly influenced by vehicle traffic. A wireless camera (DCS-920, D-Link Corporation) overlooking the investigated carpark area was installed and programmed to take a picture every five minutes verifying that no tampering occurred with the experimental set-up and that no traffic approached the unused area. | ||
| Fig. 2 Schematic of rainfall simulator (a), methodology overview (b), and experimental site (1.6 ha carpark with 650 parking lots) showing the location of constructed boards both in the busy (entrance) and unused traffic zone during the experiment (c). | ||
Rainfall simulation
A two-nozzle (Veerjet 80100) Norton type rainfall simulator21 was used to simulate rainfall for contaminant wash-off (Fig. 2a). Tap water (specific conductance after pH adjustment: 180 μS cm−1) used for the simulated rainfall was pre-filtered through a 10 μm cartridge filter to remove any solids in the water. A control sample of the feed water was taken before and after each wash-off and analyzed for heavy metals, and results (<0.5 μg L−1 for Pb, <10 μg L−1 for Cu, <40 μg L−1 for Zn) were taken into account during data analysis. The pH of the feed water was adjusted to 6.0 using concentrated nitric acid in order to simulate the pH of rainwater in Christchurch (measured on-campus during 3 rain events in 2009; see also Ref. 8 for reported rain pH in Auckland, New Zealand between 5.8 and 6.4) and maintained at pH 6.0 throughout the experiment. During pH-adjustment the pH buffering capacity of the tap water was depleted, thus making the feed water susceptible to pH changes as is the case with natural rain. All boards underwent an initial flushing with simulated rainfall by applying the highest intensity setting of 120 mm·hr−1 for 15 min prior exposure. Control samples, taken at the end of the washing step, showed that some zinc was released mainly from coarse asphalt boards (1.5 μg L−1 from concrete, 7.4 μg L−1 from smooth asphalt, 47.2 μg L−1 from coarse asphalt), but no copper, lead or TSS. After boards were deployed at the research field site for the designated time period of 7 days, they were collected (carefully covered by cardboard sheets during transport to prevent potential cross contamination) and placed under the rainfall simulator in the laboratory at an inclination of 4 degrees to generate surface runoff and hence determine contaminant accumulation rates. A rainfall intensity of 22 mm·hr−1, which equates to upper rainfall intensity in the local catchment,23 was applied for 40 min for each experimental run, resulting in a rainfall depth of 14.7 mm per experimental run which is comparable to a 2 year return period for a 2 h rain event in Christchurch, NZ. Rainfall velocity and droplet size distribution generated by the rainfall simulator and from rainfall occurring naturally near the field site under study was measured using a PARSIVEL laser disdrometer (Ott, Germany). The simulated rainfall droplet size distribution aligned well with observed natural rainfall patterns with peak intensities of 20 mm·hr−1 and a droplet size peak of 0.5 mm (Fig. 3). | ||
| Fig. 3 Average droplet size distributions of a 45-minute natural storm event (peak intensity of 20 mm h−1) compared to rain generated by the rainfall simulator at 22 mm h−1. | ||
Chemical analysis
Runoff samples from the boards were collected at 0, 5, 10, 20, and 40 min intervals following application of the simulated rainfall. Samples were instantaneously measured for pH (YSI Model 60 pH field meter) and turbidity (Hach Model 2100P portable turbidimeter) with instruments pre-calibrated on the day of the experimental runs. Heavy metals (Zn, Cu and Pb) were analyzed by ICP-MS (Agilent) following APHA Method 3125B after HNO3 digestion.24 Total metal digestions were prepared by thoroughly mixing samples on a magnetic stir plate and transferring 25 mL to a 50 mL polypropylene centrifuge tube. After the addition of 5 mL concentrated (69%) nitric acid (Fisher, trace analysis grade), tubes were placed in a heating block and samples were boiled for 1 h. Cooled samples were then filtered through an encapsulated 0.45 μm PVDF filter (47 mm, Labserv) and analyzed viaICP-MS. Total suspended solids (TSS) were measured within 24 h following APHA Method 2540D.24 Dissolved organic carbon (DOC) concentrations were analyzed using an Apollo 9000 TOC Analyzer (Teledyne Tekmar, US) with samples filtered through an encapsulated 0.45 μm PVDF filter (47 mm, Labserv) prior analysis. Quality assurance protocols including blanks, duplicates (10% of samples) and instrument calibration were conducted throughout each batch analysis. Particle size distributions in runoff were measured using a Horiba LA-950 laser scattering particle size distribution analyzer.Results and discussion
Heavy metals and TSS
Total heavy metal (Zn, Cu and Pb) and total suspended solids (TSS) concentrations measured in runoff from all three impervious surface types from both locations (unused zone of the carpark 50 m away from vehicular traffic and adjacent to the carpark entrance) over a 7-day dry period are presented in Fig. 4 and 5a/d. Both metal and TSS concentrations in runoff showed exponential decline for all contaminants and all three impervious surface types at both locations. Within 10–20 min of the simulated storm, metal and TSS concentrations decreased to <15% of their initial concentrations, demonstrating a distinct first-flush behavior as reported elsewhere.18, 19, 23 However, distinct differences between surface types can be noticed. | ||
| Fig. 4 Concentrations of zinc (a, d), copper (b, e) and lead (c, f) in runoff from concrete and asphalt boards of different roughness exposed for 7 days at two different locations (carpark entrance and unused part of a carpark >50 m away from traffic, n = 2). | ||
 | ||
| Fig. 5 TSS concentrations and turbidity (a, d), pH (b, e) and DOC concentrations (c, f) in runoff from concrete and asphalt boards of different roughness after being exposed for 7 days at two different locations (carpark entrance and unused part of a carpark >50 m away from traffic, n = 2). | ||
For TSS, higher initial concentrations of suspended particles were washed off from concrete surfaces (e.g. 87 mg L−1TSS at carpark entrance) compared with the rough (42 mg L−1) or smooth (27 mg L−1) asphalt surfaces (Fig. 5a and d). Different surface roughness between the three paving materials is considered to have influenced particle (i.e.TSS) dislodgement. Particles deposited on the smooth concrete surface with a standard deviation in elevation of just 0.13 mm can be more easily dislodged during wash-off compared to the rougher asphalt surfaces (4 and 6 times higher standard deviation in elevation caused by cavities; Fig. 1), whose deeper cavities and greater adhesion of the material prevented particles from being washed off. This implies that during wash-off a certain proportion of deposited particles is held back in cavities of both asphalt types leading to the observed lower TSS concentrations in runoff. Comparison between different asphalt types showed higher TSS concentrations in runoff from coarse (14 mm) compared to smooth (3 mm) asphalt, indicating that during time of atmospheric exposure of the boards particles become more easily entrapped in the deeper cavities of the coarse asphalt, and thus more particles can accumulate and be washed off compared to the smooth asphalt surface.
Maximum total heavy metal concentrations at t = 0 (first runoff immediately following simulated rainfall application) from boards exposed at the carpark entrance were 982 μg Zn L−1 and 73.5 μg Cu L−1 from coarse asphalt and 11.3 μg Pb L−1 from concrete surfaces (Fig. 4d–f). Runoff from boards exposed 50 m away from any vehicular traffic yielded maximum total metal concentrations at t = 0 from the same surface types at 733 μg Zn L−1 and 50.8 μg Cu L−1 from coarse asphalt and 20.7 μg Pb L−1 from concrete surfaces (Fig. 4a–c). Initial metal concentrations were compared with the effects-based Australian and New Zealand Environment and Conservation Council (ANZECC) guidelines relevant for fresh and marine water quality showing that these concentrations exceeded the guideline thresholds several-fold (e.g. 90% trigger value for zinc of 15 μg L−1 is exceeded 65-fold at 982 μg L−1, 90% trigger value for copper of 1.8 μg L−1 is exceeded 40-fold at 73.5 μg L−1). Although these initial high concentrations quickly drop during the time of runoff and are further diluted in receiving streams, their exceedance magnitudes can impose adverse effects on receiving ecosystems when directly discharged to streams, especially when stormflow volumes are high compared to baseflow conditions. This has been demonstrated in a study showing high metal toxicity in highway runoff during initial storm durations.10 The same study also identified zinc and copper as the main toxicants.
Comparison of metal concentrations in relation to the impermeable surface types revealed differences in metal-specific behavior between concrete and asphalt compositions. Total lead concentrations were higher in runoff from concrete surfaces compared to both asphalt types (Fig. 4c and f). As lead is mainly particle-bound in stormwater runoff (e.g.18 and 25), this is consistent with findings of higher TSS concentrations in runoff from concrete boards as described above. Zinc and copper concentrations, however, were mostly higher in runoff from both asphalt surface types compared to concrete runoff (Fig. 4), which can be explained by a combination of two effects. Firstly, the deeper cavities in the asphalt surfaces (Fig. 1) initially afford greater entrapment of particulate metals during time of atmospheric exposure leading to higher cumulative total amounts for Zn and Cu deposited on the asphalt boards. Although these particulate metals are washed off incompletely as indicated by TSS results (see above), the much lower pH in asphalt runoff (average pH: 5.9) compared to concrete board runoff (average pH: 9.7) may then have facilitated metal dissolution from these entrapped particles once wash-off commenced. As shown in studies investigating metal speciation in stormwater (e.g.18 and 25), Zn and Cu are predominantly in a dissolved state at a pH of 6 that is applied in this study. Consequently, higher Zn and Cu concentrations (dominated by dissolved forms) would wash off from asphalt compared to concrete surfaces. Conversely, a higher pH in concrete runoff could have facilitated metal complexation (rather than dissolution) to the concrete surface as was demonstrated for copper by Wallinder et al.26
Contaminant yields
Contaminant yields (mass m−2 d−1) were calculated for all boards from runoff concentration profiles and runoff volumes and ranged from 18–49 mg m−2 d−1 for total particulates (TP), 140–440 μg m−2 d−1 for zinc, 16–29 μg m−2 d−1 for copper and 2–12 μg m−2 d−1 for lead (Table 1). These values compare well with pollutant fluxes of urban background and low volume roads (<2,000 vehicles per day) determined in a recent study on atmospheric deposition in an urban catchment within the Sydney metropolitan area (19–58 mg m−2 d−1 for TP, 96–238 μg m−2 d−1 for zinc, 11–28 μg m−2 d−1 for copper and 7–22 μg m−2 d−1 for lead,7) and demonstrate that substantial amounts of particulate contaminants (equating e.g. to 66–179 kg ha−1 yr−1 TP and 0.5–1.6 kg ha−1 yr−1 zinc) are deposited in urban areas at low traffic locations.| Yields per day - total metals [mg m−2/d] | ||||||
|---|---|---|---|---|---|---|
| Smooth asphalt (3 mm) | Coarse asphalt (14 mm) | Concrete | ||||
| Entrance | 50m away | Entrance | 50m away | Entrance | 50m away | |
| Total particulates | 18.2 ± 3.2 | 19.9 ± 5.4 | 25.9 ± 3.0 | 28.2 ± 3.3 | 29.2 ± 1.5 | 49.4 ± 6.0 |
| Zinc | 0.33 ± 0.12 | 0.30 ± 0.05 | 0.44 ± 0.05 | 0.38 ± 0.06 | 0.14 ± 0.04 | 0.15 ± 0.03 |
| Copper | 0.022 ± 0.004 | 0.016 ± 0.004 | 0.029 ± 0.006 | 0.022 ± 0.009 | 0.020 ± 0.002 | 0.027 ± 0.003 |
| Lead | 0.004 ± 0.001 | 0.002 ± 0.0004 | 0.005 ± 0.002 | 0.008 ± 0.003 | 0.008 ± 0.001 | 0.011 ± 0.002 |
Comparison between both locations of exposure show that boards placed at least 50 m away from vehicle traffic had daily yields for TSS, zinc and copper that were similar (mostly within 25%), sometimes even higher (e.g.TSS and copper yields from concrete surfaces) compared to boards exposed at the carpark entrance (Table 1). This was initially unexpected as about 3250 cars were counted passing the carpark entrance in both directions during time of exposure (∼600 during weekdays, 250 over the weekend), which was expected to have contributed to a higher heavy metal deposition on boards exposed adjacent to the access road to the carpark. However, since results showed that the contribution of heavy metals from cars passing the carpark entrance was mostly small (comparing contaminant accumulation at both sites, see Table 1), it is now hypothesized that contaminants from main roads surrounding the study site (with traffic volumes of 13,000–15,000 vehicles/d at distances of 100–300 m, and 32,000 vehicles/d 600 m away27) have considerably contributed to the contaminant yields collected on the boards at both locations. This would mean that substantial atmospheric distribution of heavy metal and TSS particulates over greater distances from traffic occurred.
Distribution of particulate contaminants is dependent on the particle size distribution, as small particles are more susceptible to be transported over longer distances.28,29 Particle size analysis of initial runoff from concrete surfaces (chosen as most representative sample for particle size distribution as runoff of particulates from this smoothest surface type has been shown to be least hindered e.g. by cavities) showed a high percentage of small particles susceptible to atmospheric dispersion over longer distances with 28% of the particulates (cumulative volume fraction) <10 μm, 39% <20 μm and 60% <50 μm. This supports the hypothesis that particulate contaminants deposited on the boards can be transported over longer distances.
Atmospheric transport of stormwater contaminants has also been demonstrated by Sabin et al.,16 who showed that 52% of zinc and 70% of copper in stormwater runoff within a small urban catchment originated from dry atmospheric deposition. More recently, Brett and Birch (2011) investigated the spatial distribution of bulk atmospheric deposition of heavy metals in Sydney metropolitan area and attributed 70–80% of their total atmospheric flux to vehicular activity.7 They also reported increased background levels of atmospheric heavy metal depositions in urban areas for the Sydney Metropolitan area (e.g. 100–400 μg m−2 d−1zinc or 5–25 μg m−2 d−1copper at two background locations at least 500 m away from the nearest major road). Furthermore, Sabin et al.17 showed that traffic-related contaminants from vehicle emissions at a freeway in Los Angeles (300,000 vehicles/day) were transported between 150–450 m downwind, with atmospheric total suspended particle concentrations of zinc, copper and lead still constituting approximately half the concentration at a distance of 450 m compared to samples collected only 10 m away. In contrast to these studies in which grease plates or cylindrical plastic tanks were used to capture particulates, contaminants in this study were deposited directly on impervious surface types typical of the urban catchment, allowing a better representation of accumulation and wash-off processes. However, the results of the above cited studies nevertheless support the assumption derived from our data that heavy metal contaminants in urban areas can be distributed over larger areas and not only in proximity to major roads. This would subsequently lead to increased background levels of atmospheric heavy metal depositions in urban areas as e.g. shown by Brett and Birch (2011) for the Sydney Metropolitan area7 (see above).
Other water quality parameters
Other water quality parameters measured in board runoff beside TSS and heavy metal concentrations were pH (being the most important parameter influencing metal leachability and speciation), dissolved organic carbon (DOC) and turbidity (Fig. 5).The pH in runoff from concrete over the entire simulated storm event of 40 min was consistently almost four pH units higher (average: 9.7) than asphalt runoff (average: 5.9) or the applied rain water pH of 6.0 (Fig. 5b and e). This was quite surprising as boards were constructed about 8 months prior the experiments with ∼10 previous wash-off events. A higher pH in concrete runoff likely resulted from dissolution of calcium hydrates such as Ca(OH)2 at the concrete surface, which is previously reported by Athanasiadis et al.30 who observed a pH increase of three units (from 7.0 to 10.0) in water stored in a concrete tank for 15 min. The pH increase can lead to complexation and hence reduction of the bioavailable (i.e. dissolved) copper fraction.26,31 This suggests some benefits may exist in employing concrete pipes (compared with other materials) to convey untreated stormwater discharge to neighboring waterways when necessary.26 The runoff pH from both coarse and smooth asphalt surfaces was very similar, between 5.5 and 6.0 during the experiments. Since the feed rain water was maintained at a constant pH of 6.0 (and its buffering capacity being depleted during pH-adjustment), the slightly lower pH observed during the initial 10 min of runoff (Fig. 5b) may have resulted from metal hydrolyzation during initial surface wash-off.
Dissolved organic carbon (DOC) has the potential for complexation of dissolved metals (especially copper) through formation of DOC-metal complexes which can influence metal mobility, as organic ligands in solution may compete with the adsorption of dissolved metals to solid surfaces.32 Furthermore, increasing DOC concentration can reduce toxicity of dissolved copper and zinc.33 High initial DOC concentrations in runoff from both asphalt type boards (83–85 mg L−1 from coarse and 52–55 mg L−1 from smooth asphalt) compared to concrete board runoff (7 and 19 mg L−1) as shown in Fig. 5c and 5f were attributed to greater amounts of vegetative material (e.g. pollen and small seeds) that were visually observed in the cavities of the asphalt surfaces before wash-off. The sharp exponential decline in DOC concentration within the first 10–20 min of wash-off reflects the consistent initial wash-off and stabilization patterns observed for metals (Fig. 4) and the other water quality parameters (Fig. 5). Average DOC concentrations (7.8 mg L−1 for concrete runoff, 20.1 mg L−1 for smooth asphalt runoff and 30.3 mg L−1 for coarse asphalt runoff) are higher for asphalt surfaces and lower for concrete runoff compared to an extensive study of highway runoff in California34 that reported a mean DOC concentration of 13.0 mg L−1 for non-urban highways (<30,000 vehicles per day).
Turbidity, a rapid, cheap and loggable instantaneous optical measure of the concentration of suspended particles in a solution, was positively correlated with the more cumbersome, expensive and non-loggable TSS concentrations. This is represented by the linear relationship TSS [mg L−1] = 1.5 · Turbidity [NTU] (R2 = 0.89, n = 60). Establishing relationships between turbidity and suspended solids measurements can help provide a more instantaneous, loggable and cost-effective estimation of solids concentration in surface waters. Although such relationships are site-specific, once established for a catchment of interest, turbidity measurements can be employed as a surrogate measurement of suspended solids and even other related water quality parameters.35,36
Conclusions
The experimental approach of deploying portable boards to investigate urban contaminant accumulation on different impervious surfaces was successfully employed to quantify atmospheric deposition and wash-off rates of contaminants through measurements of heavy metal, suspended solids and other water quality parameters. Contaminant yields with respect to different surface characteristics and spatial location were determined allowing specific parameter effects to be investigated, which is not typically afforded in other stormwater monitoring approaches. Results indicate that particulate metal contaminants originating in one catchment could actually be deposited further afield in a neighboring catchment raising further complex challenges regarding stormwater management, its mitigation and liability. In addition, distinct differences in contaminant accumulation and wash-off characteristics between concrete and asphalt surfaces were demonstrated and attributed to material surface characteristics (roughness caused by cavities, adhesiveness) as well as substantial differences of runoff pH between both surface types.Clear advantages of the methodology described in this study include the possibility to easily examine multiple locations within a catchment simultaneously by deploying boards with surfaces of interest within different parts of a catchment to understand spatial contaminant transport patterns. Use of a rainfall simulator under controlled lab conditions enables researchers to investigate specific parameters influencing contaminant behavior such as pavement composition, rainfall intensity, storm duration or feed water chemistry (e.g. rain pH). The method can be used to develop contaminant build-up and wash-off coefficients for different pavement types that are required for stormwater models (e.g. the US EPA SWMM).
Notes and references
- P. Gobel, C. Dierkes and W. C. Coldewey, J. Contam. Hydrol., 2007, 91, 26–42 CrossRef CAS.
- M. Clara, G. Windhofer, W. Hartl, K. Braun, M. Simon, O. Gans, C. Scheffknecht and A. Chovanec, Chemosphere, 2010, 78, 1078–1084 CrossRef CAS.
- D. S. T. Hjortenkrans, B. G. Bergback and A. V. Haggerud, Environ. Sci. Technol., 2007, 41, 5224–5230 CrossRef CAS.
- G. F. Birch and L. Rochford, Environ. Monit. Assess., 2010, 169, 531–551 CrossRef CAS.
- J. Sternbeck, A. Sjodin and K. Andreasson, Atmos. Environ., 2002, 36, 4735–4744 CrossRef CAS.
- B. Bergbäck, K. Johansson and U. Mohlander, Water, Air, Soil Pollut.: Focus, 2001, 1, 3–24 CrossRef.
- B. S. Davis and G. F. Birch, Water, Air, Soil Pollut., 2011, 214, 147–162 CrossRef CAS.
- S. L. Pennington and J. G. Webster-Brown, New Zealand Journal of Marine and Freshwater Research, 2008, 42, 99–108 CrossRef CAS.
- C. Karlen, I. O. Wallinder, D. Heijerick, C. Leygraf and C. R. Janssen, Sci. Total Environ., 2001, 277, 169–180 CrossRef CAS.
- M. Kayhanian, C. Stransky, S. Bay, S. L. Lau and M. K. Stenstrom, Sci. Total Environ., 2008, 389, 386–406 CrossRef CAS.
- D. Greenstein, L. Tiefenthaler and S. Bay, Arch. Environ. Contam. Toxicol., 2004, 47, 199–206 CrossRef CAS.
- G. Beasley and P. Kneale, Progress in Physical Geography, 2002, 26, 236–270 CrossRef.
- P. Mahbub, G. A. Ayoko, A. Goonetilleke, P. Egodawatta and S. Kokot, Environ. Sci. Technol., 2010, 44, 8904–8910 CrossRef CAS.
- C. Zoppou, Environ. Modell. Software, 2001, 16, 195–231 CrossRef.
- N. Seelsaen, R. McLaughlan, S. Moore and R. M. Stuetz, Water Sci. Technol., 2006, 54(6–7), 299–305 CAS.
- L. D. Sabin, J. H. Lim, K. D. Stolzenbach and K. C. Schiff, Water Res., 2005, 39, 3929–3937 CrossRef CAS.
- L. D. Sabin, J. H. Lim, M. T. Venezia, A. M. Winer, K. C. Schiff and K. D. Stolzenbach, Atmos. Environ., 2006, 40, 7528–7538 CrossRef CAS.
- J. J. Sansalone and S. G. Buchberger, J. Environ. Eng., 1997, 123, 134–143 CrossRef CAS.
- J. H. Lee, K. W. Bang, L. H. Ketchum, J. S. Choe and M. J. Yu, Sci. Total Environ., 2002, 293, 163–175 CrossRef CAS.
- P. Egodawatta, E. Thomas and A. Goonetilleke, Water Res., 2007, 41, 3025–3031 CrossRef CAS.
- L. Herngren, A. Goonetilleke and G. A. Ayoko, J. Environ. Manage., 2005, 76, 149–158 CrossRef CAS.
- F. Darboux and C. Huang, Soil Sci. Soc. Am. J., 2003, 67, 92–99 CrossRef CAS.
- J. M. Zollhoefer, First flush stormwater: time series and event mean concentrations, 6th South Pacific Stormwater Conference, 29 April–1 May 2009, Auckland, New Zealand Search PubMed.
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association. 21st Edition, Washington DC, 2005 Search PubMed.
- L. M. Mosley and B. M. Peake, New Zealand Journal of Marine and Freshwater Research, 2001, 35, 615–624 CrossRef CAS.
- I. O. Wallinder, Y. Hedberg and P. Dromberg, Water Res., 2009, 43, 5031–5038 CrossRef.
- Road Assessment, Management & Maintenance (RAMM) data from the Crash Analysis System (CAS) accessed in July 2011, NZ Transport Agency, Wellington, New Zealand Search PubMed.
- J. F. Leys and G. H. McTainsh, Earth Surf. Processes Landforms, 1996, 21, 661–671 CrossRef CAS.
- B. Sharratt, Soil Sci. Soc. Am. J., 2011, 75, 1054–1060 CrossRef CAS.
- K. Athanasiadis, B. Helmreich and H. Horn, Water Res., 2007, 41, 3251–3258 CrossRef CAS.
- B. Bahar, G. Herting, I. O. Wallinder, K. Hakkila, C. Leygraf and M. Virta, Environ. Monit. Assess., 2008, 140, 175–189 CrossRef CAS.
- Y. Mason, A. A. Ammann, A. Ulrich and L. Sigg, Environ. Sci. Technol., 1999, 33, 1588–1597 CrossRef CAS.
- R. V. Hyne, F. Pablo, M. Julli and S. J. Markich, Environ. Toxicol. Chem., 2005, 24, 1667–1675 CrossRef CAS.
- M. Kayhanian, C. Suverkropp, A. Ruby and K. Tsay, J. Environ. Manage., 2007, 85, 279–295 CrossRef CAS.
- N. R. Thomson, E. A. McBean, W. Snodgrass and I. B. Monstrenko, Water Air Soil Poll., 1997, 94, 307–347 CAS.
- M. B. Henjum, R. M. Hozalski, C. R. Wennen, W. A. Arnold and P. J. Novak, J. Environ. Monit., 2010, 12, 225–233 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
