TNT removal from culture media by three commonly available wild plants growing in the Caribbean
Sandra N.
Correa-Torres
,
Leonardo C.
Pacheco-Londoño
,
Eduardo A.
Espinosa-Fuentes
,
Lolita
Rodríguez
,
Fernando A.
Souto-Bachiller
and
Samuel P.
Hernández-Rivera
*
ALERT DHS Center of Excellence, Department of Chemistry, University of Puerto Rico-Mayagüez, Mayagüez, PR 00681, Puerto Rico. E-mail: samuel.hernandez3@upr.edu
First published on 24th November 2011
Abstract
Plants growing in the Caribbean, Rubia tinctorum, Lippia dulcis and Spermacoce remota, were used in vitro to remove TNT from culture media. Plants were found to be resistant to high TNT levels. S. remota was able to remove TNT in less than 48 h. Part of the TNT was physically removed from the culture media by evaporation.
Environmental impactThe remediation of nitroaromatic compounds in natural waters and soils is a subject of considerable current interest. Phytoremediation is a very effective “green” technique in which soil functions are maintained and life in the soil is reactivated. However, plant tolerance to contaminants depends on the plant species, the bioavailability and soil characteristics, and the plant chemo-type and ecotype. Therefore, there is a need to identify new plants that are capable of participating in different phytoremediation schemes. Here, we report three plants commonly available in the Caribbean, Rubia tinctorum, Lippia dulcis and Spermacoce remota, that have been successfully used in vitro to remove TNT from culture media. S. remota was able to remove TNT in less than 48 h, with partial degradation to dinitrotoluenes and to amino-dinitrotoluenes. |
1. Introduction
High explosives (HEs) and their decomposition products are among the principal contributors to soil and water pollution resulting from manufacture, use and disposal.1,2 The important HE, 2,4,6-trinitrotoluene (TNT), is a good example of a pollutant that has attracted considerable attention in recent years due to its wide use as a military explosive. It is a highly persistent mutagen classified as a Group C human carcinogen that threatens human health.3 Besides constituting a major component of about 80% of landmines, it is found as a contaminant at military sites in Europe and USA as a result of munitions manufacturing, handling and disposal operations carried out since World War II. TNT slowly degrades in contaminated soils, sediments and natural waters. The main degradation products found in contaminated soils are 2-amino-4,6-dinitrotoluene (2-A-4,6-DNT), 4-A-2,6-DNT, 2,4-diamino-6-nitrotoluene (2,4-DA-6-NT), 2,6-DA-4-NT and aromatic hydroxylamines.4–7 Remediation technologies for TNT contaminated soils include incineration, land filling and composting and microbial and fungal bioremediation. In contrast to conventional technologies, phytoremediation uses plants to remove pollutants and offers several benefits: effective, inexpensive, in situ and “green” i.e. soil functions are maintained and life in soil is reactivated.3,8–10 Plants tolerance to contaminants depends on species, growth stage, bioavailability, soil characteristics and plant chemo-type and ecotype. Ramos et al. reported that wild plants showed limited tolerance to aqueous media with 1–5 mg L−1TNT.11 Plants showed low tolerance to TNT and suffered from chlorosis and died. This motivated this study with plants commonly available in the Caribbean: Rubia tinctorum, Lippia dulcis and Spermacoce remota.12,13 These plants tolerated well higher levels of TNT (2.5–35 mg L−1), and most importantly, S. remota was able to remove TNT in less than 48 h, with partial degradation to DNTs and to ADNTs.2. Materials and methods
Crystalline TNT (99% purity, 30% min. water) was obtained from ChemService, Inc. (West Chester, PA). HPLC grade methanol and water were acquired from Sigma-Aldrich Chemical Co. (Milwaukee, WI). Analytical standards for TNT and target degradation products (Mix A) were purchased from Restek Corp. (Bellefonte, PA) with each compound at a concentration of 1 mg·mL−1. GC grade standard TNT solutions were also obtained from Restek Corp. The plant culture medium used was a modified Murashige and Skoog (M&S) with basal salt media and macro- and micro-nutrients, acquired from Sigma-Aldrich.14SPME based methodologies for HE analysis were as described previously.15,16HPLC analyses were carried out in an Agilent Technologies chromatograph (model 1100) equipped with a diode-array UV-VIS detector.17GC analyses for TNT evaporation rate studies were carried out with an Agilent Technologies model 6890N Network GC System, equipped with a 63Ni micro-cell electron capture detector (μECD). GC separations were based on EPA Method 8095 with ECD as the GC detector.18
Culture media and instruments for in vitro procedures were sterilized in an autoclave (Sterilmatic, Market Forge). All experiments were carried out in a laminar flow hood. A custom culture chamber manufactured by Alliance Industries, California, was used for all plant growth and phytoremediation experiments. This chamber is fitted with HEPA filters and provided full control of temperature, humidity, photoperiod and irradiance. All experiments were carried out with an irradiance of 1000 lux, at 25 °C, with 16 h light/8 h dark period unless otherwise stated. L. dulcis and R. tinctorum were 4-week old plantlets from the stock of axenic germplasm. The L. dulcis stock is from a population located in Sector Toro Negro, Orocovis, Puerto Rico (specimen deposited at the Herbarium of the Biology Department, UPR-M). The stock has been maintained since 1990 by clonal micropropagation using single node culture in semisolid nutrient medium (0.8% agar, full strength M&S basal salts, 3% sucrose, pH 5.8).19 The R. tinctorum stock is from natural seeds provided by the Richters Herb Specialists, Goodwood, Ontario, Canada (Madder: S3830, Lot: 15404, Origin: NL). Seeds were hydrated and cleaned in Petri dishes, peeled and chemically sterilized. Upon germination in wetted filter paper in the dark, seedlings were cultured in test tubes in semisolid nutrient medium (0.5% agar, full strength M&S basal salts, 5% sucrose, pH 5.8). The healthiest plantlets were selected and used to establish a clonal stock that is maintained by single node micropropagation using the same culture conditions as for L. dulcis. S. remota is from a population found in the same location as L. dulcis (specimen deposited at the UPR-M Herbarium). Botanical enquiries led to a plant known by the vernacular name “Juana la Blanca”. This plant was initially identified as Borreria laevis (Lam.) Griseb, but since there is another plant, Spermacoce confuse, of the same family and same vernacular name, a herbarium specimen was sent to the Missouri Botanical Garden (Saint Louis, MO) where it was finally identified as Spermacoce remota Lam. Fresh wild plants were cultured in greenhouse and used as explant source to establish a stock of axenic plantlets (0.8% agar, full strength M&S basal salts, 3% sucrose, 2 mg L−1indole-3-acid acetic, pH 5.8) suitable for phytoremediation experiments described.20
TNT removal experiments were conducted in liquid culture media. Stock solutions were prepared dissolving TNT in M&S sterile medium. Work solutions were prepared from the stock to a final TNT concentration of 2.5, 35 and 75 mg L−1. Four-week old plantlets were transferred to 125 mL Erlenmeyer flasks containing culture media and TNT. Two kinds of blanks were prepared: (1) control with culture medium and plants but without TNT and (2) control with culture medium and TNT but without plants. Erlenmeyer flasks were placed in the culture chamber on a platform orbital shaker at 100 rpm. All flasks were sampled at timed intervals by removing 150 μL aliquots for HPLC analysis. Samples were analyzed in quintuplicate until the TNT concentrations decreased below undetectable levels.
Photodegradation and evaporation experiments were done to take into account these two abiotic processes of TNT removal from aqueous solutions. Six Erlenmeyer flasks with 30 mL of M&S medium containing 32 mg L−1 of TNT were placed for 30 days in the culture chamber. Three of these were exposed to visible light and the other three remained in the dark. Samples were analyzed for their remaining TNT concentrations. For evaporation experiments, the TNT vapor in the head space of the culture medium was monitored using HS-SPME for sample concentration and collection. Samples were prepared in 15 mL vials with 10 mL of M&S medium containing 35 and 75 mg L−1 of TNT. The SPME fibers were introduced into the vial and placed 1 cm above the vapor–liquid interface. The extraction time in the head space of the samples was fixed at 5 min. These samples remained at constant agitation within the environmental chamber for the test period. Sampling was carried out within 30 min to 48 h after sample preparation.
3. Results and discussion
The three plant species used for this work, R. tinctorum, L. dulcis and S. remota, were selected on the basis of their natural availability and potential robustness for their prospective cultivation at contaminated Caribbean military sites. We have found that all three plants are tolerant to TNT levels higher than 5 mg L−1 when grown in aqueous culture media. Prior to this finding, no report was available indicating that the phytoremediation potential of these plants had been studied. L. dulcis and R. tinctorum were available from the stock of axenic germplasm maintained at the Natural Products Laboratory (NPL, UPRM). The in vitro culture of S. remota had not been described in the literature prior to this work and is herein reported in a preliminary form (see Experimental section).R. tinctorum removed nearly 100% of the TNT in liquid medium after 400 h (17 days). This plant was consistently tolerant to TNT concentrations of up to 35 mg L−1. Exposed plantlets were healthy, did not suffer from chlorosis, and showed no symptoms of stem bending or any other stress. Fig. 1a displays a typical TNT removal profile. The TNT concentrations declined in a nearly first-order process with a half-life of 34 h, whereas the control blanks showed zero-order kinetics with a half-life of 361 h. This represents more than a ten-fold improvement in TNT removal capacity that can be attributed to the plant.
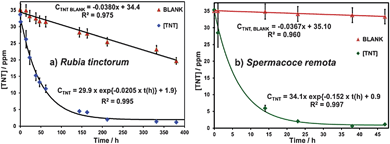 | ||
| Fig. 1 TNT removal profiles of plants studied: (a) R. tinctorum; (b) S. remota. The initial concentration of TNT was 35 mg L−1. | ||
The zero-order kinetics observed for the TNT blanks is consistent with losses by a physical evaporation process. Evaporation would be independent of the TNT concentration and depends mainly on the TNT vapor pressure in the head space of the non-hermetically capped tissue culture flasks. Similar results (not shown) were obtained with L. dulcis plantlets. TNT concentrations decline also followed first-order kinetics with a half-life of 98 h. In contrast, when S. remota was exposed to TNT under identical conditions, the TNT in solution was removed in less than 48 h. Fig. 1b is a representative removal profile with a half-life of 4.5 h.
Additional experiments were done to characterize possible photo-degradation and evaporation of TNT under the plant culture conditions. Erlenmeyer flasks with 30 mL of M&S medium containing 32 mg L−1 of TNT were placed for 30 days in the culture chamber. Samples were analyzed by HPLC and GC for their remaining TNT and possible degradation products. Light-induced degradation of TNT was an important concern since it has been well documented that UV light photodegrades nitroaromatic compounds. However, since degradation products were not detected in the control tests, it can be safely concluded that there were no TNT losses due to photodegradation in the plant culture experiments herein reported.
The decline in TNT concentration observed for the blanks was clearly strong evidence that a physical evaporation process was taking place. TNT removal rates calculated for each plant took into account mass losses in the blanks. However, direct demonstration of physical evaporation of TNT from solution entailed measurement of vapor density in the head space of the blanks. Sampling of the head space over culture medium was accomplished using HS-SPME (sample concentration/collection). Fig. 2 shows the TNT evaporation profile in liquid medium as determined using GC/μ-ECD. Initially, the evaporation process was monitored by HS-SPME because the amount of TNT adsorbed was proportional to the concentration in the gas phase. Evidently, TNT vapors saturate the head space in ≤5 h.
 | ||
| Fig. 2 Vapor concentration in head space over TNT solution of initial concentration of 35 mg L−1 detected by HS-SPME using GC/μ-ECD. Samples were analyzed at an extraction time of 5 min. | ||
The fate of the TNT added to the culture media was investigated for the three plants. Firstly, the culture media themselves were analyzed by HPLC as a function of time noting the TNT decline kinetics. Degradation products have been tentatively identified as 2-A-4,6-DNT, 4-A-2,6-DNT, 2,4-DNT and 2,6-DNT. The data are consistent with rapid TNT root absorption and plant uptake. This is followed by plants transformation into products that appear as root exudates in the medium, where they seem to be transformed further into the persistent products observed.
R. tinctorum and L. dulcis did not show any transformation products in their respective culture media as a result of TNT decline. Once the respective TNT exposure times ended, plant material from all three plants was collected, freeze dried, chopped and milled for micro-Soxhlet extraction and HPLC and GC analysis. Extracts from L. dulcis and R. tinctorum showed both the presence of residual TNT and amino and dinitro degradation products. Surprisingly enough, S. remota was clean and within experimental error showed no trace of xenobiotic organic materials. In the absence of plants, TNT was removed from the medium by evaporation. In the presence of plants, absorption and biotransformations contribute to TNT fate.
The most significant finding of this work is a 100-fold increase in TNT removal capacity for S. remota compared to a 10-fold increase for R. tinctorum and L. dulcis. At present we are investigating the nature of the enhanced behavior of S. remota. TNT was absorbed directly from liquid medium, as do non-vascular macroalgae.21 It appears that S. remota takes up TNT through epidermal cells, biotransforming and excreting products in much the same way that freshwater vascular aquatic plants and macroalgae do.21
Conclusions
TNT depletion observed in culture media of plants studied results from uptake and biotransformation into degradation products. The plants studied were tolerant to TNT without showing chlorosis of leaves, bending of stems or any other symptoms of stress. R. tinctorum and S. remota tolerated TNT well up to 37 mg L−1 whereas L. dulcis tolerated well up to 75 mg L−1.TNT uptake profiles followed first order kinetics for the plants studied. Verification of first order of uptake kinetics required measuring TNT depletion for several half-lives and correcting for losses due to evaporation in the culture flasks which was confirmed as a zero-order process. After approximately 10 h, the head space above liquid culture media flasks was saturated with TNT vapors. Evaporation losses take place even though flasks were capped with tissue culture stoppers, which fit snugly but are designed to allow gas exchange. A steady state was reached in the stoppered flasks where the TNT concentration in the head space remained constant and gave rise to observed zero-order kinetics, i.e. a rate independent of TNT concentration in the liquid media.
It is finally concluded that the three plants studied are excellent candidates for TNT removal in aqueous media and have a potential for further phytoremediation studies that mimic TNT-contaminated soils. Work is in progress to explain the biochemical reason for the higher TNT removal kinetics found for S. remota compared to the other two plants.
Acknowledgements
Authors acknowledge support from DOD MURI Program grant # DAAD19-02-1-0257 and DHS COE # 2008-ST-061-ED0001.References
- J. Jenkins, M. Walsh, D. Leggett and M. Paul, Tech. Report ERDC TR-00-5, U.S. Army Cold Regions Research and Engineering Laboratory, 2000.
- T. Frische and H. Höper, Chemosphere, 2003, 50, 415 CrossRef.
- K. Makris, K. Shakya, R. Datta, D. Sarkar and D. Pachanoor, Environ. Pollut., 2007, 146, 1 Search PubMed.
- M. Alexander, Biodegradation and Bioremediation, Academic Press, San Diego, CA, 2nd edn, 1999 Search PubMed.
- B. E. Rittman and P. L. McCarty, Environmental Biotechnology: Principles and Applications, McGraw-Hill, Boston, MA, 2000 Search PubMed.
- T. Lewis, D. Newcombe and R. Crawford, J. Environ. Manage., 2004, 70, 291 Search PubMed.
- M. Subramanian, D. Oliver and J. Shanks, Biotechnol. Prog., 2006, 22, 208 Search PubMed.
- K. E. Dalgren, S. Waara, A. Duker, T. Kronhelm and P. A. W. van Hees, Water, Air, Soil Pollut., 2009, 202(1–4), 301 Search PubMed.
- R. Bhadra, R. Spanggord, D. Wayment, J. Hughes and J. Shanks, Environ. Sci. Technol., 1999, 33, 3354 CrossRef CAS.
- S. Pavlostathis, K. Comtock, M. Jacobson and M. Saunders, Environ. Toxicol. Chem., 1998, 17(11), 2266 CrossRef CAS.
- J. L. Ramos, P. Gonzalez, A. Caballero and P. Dillewijn, Curr. Opin. Biotechnol., 2005, 16, 275 CrossRef CAS.
- H. A. Liogier, Descriptive flora of Puerto Rico and adjacent islands, 1997, University of Puerto Rico Press, San Juan, PR Search PubMed.
- E. Núñez, Plantas Medicinales de Puerto Rico (Medicinal Plants of Puerto Rico), 1989, Editorial Universidad de Puerto Rico Search PubMed.
- T. Murashige and F. Skoog, Physiol. Plant., 1962, 15(3), 473 CAS.
- S. N. Correa, B. Baez, M. de Jesus-Echevarria, M. E. Castro and S. P. Hernández-Rivera, Proc. SPIE–Int. Soc. Opt. Eng., 2005, 5794, 1272 Search PubMed.
- B. Baez, S. N. Correa and S. P. Hernandez-Rivera, Proc. SPIE–Int. Soc. Opt. Eng., 2005, 5794, 1263 Search PubMed.
- USA EPA, Method 8330, Nitroaromatics and Nitroamines by High-Performance Liquid Chromatography, IVD SW-846, USAEPA-65 FR 70678, Washington, DC, 1997 Search PubMed.
- USA EPA, Method 8095, Explosive by Gas Chromatography, EPA, Washington, DC, 2000, SW-846 Revision 0 Search PubMed.
- F. A. Souto, M. de Jesús-Echevarría, O. Cárdenas, M. Acuña, P. Meléndez and L. Romero, Phytochemistry, 1077, 44(6) Search PubMed.
- R. Pierik, In Vitro Culture of Higher Plants, Martinus Nijhoff Publishers, Boston, MA, 1987 Search PubMed.
- O. Cruz-Uribe, D. Cheney and G. Rorrer, Chemosphere, 2007, 67, 1469 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |
