Superior CO2 uptake of N-doped activated carbon through hydrogen-bonding interaction†
Abstract
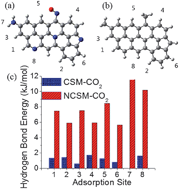
Here we show that the introduction of N into a carbon surface facilitates the hydrogen-bonding interactions between the carbon surface and CO2 molecules, which accounts for the superior CO2 uptake of the N-doped activated carbons. This new finding challenges the long-held viewpoint that acid–base interactions between N-containing basic functional groups and acidic CO2 gas are responsible for the enhanced CO2 capture capacity of N-doped carbons.
- This article is part of the themed collection: Carbon Dioxide

 Please wait while we load your content...
Please wait while we load your content...