 Open Access Article
Open Access ArticleSustainability assessment of novel chemical processes at early stage: application to biobased processes†
Akshay D.
Patel
*a,
Koen
Meesters
b,
Herman
den Uil
c,
Ed
de Jong
d,
Kornelis
Blok
a and
Martin K.
Patel
*a
aEnergy and Resources, Copernicus Institute of Sustainable Development, Utrecht University, Budapestlaan 6, 3584 CD Utrecht, The Netherlands. E-mail: a.d.patel@uu.nl; m.k.patel@uu.nl
bWageningen University of Research, Wageningen, The Netherlands
cEnergy research Center of the Netherlands, Petten, The Netherlands
dAvantium Chemicals B.V., Amsterdam, The Netherlands
First published on 14th June 2012
Abstract
Chemical conversions have been a cornerstone of industrial revolution and societal progress. Continuing this progress in a resource constrained world poses a critical challenge which demands the development of innovative chemical processes to meet our energy and material needs in a sustainable way. This challenge forms the basis for this article. We report a method for quick preliminary assessment of chemical processes at the laboratory stage. The proposed method enables a review of chemical processes within a broader sustainability context. It is inspired by green chemistry principles, techno-economic analysis and some elements of environmental life-cycle assessment (LCA). This method evaluates a proposed chemical process against comparable existing processes using a multi-criteria approach that integrates various economic and environmental indicators. An effort has been made to incorporate quantitative and qualitative information about the processes while making the method transparent and easy to implement based on information available at an early stage in process development. The idea is to provide a data-based assessment tool for chemists and engineers to develop sustainable chemistry. This paper describes the method in detail and examines plausibility of the results. A biobased process for the production of but-1,3-diene has been analyzed using this method. This biobased process is compared with a conventional process for the production of but-1,3-diene from petroleum sources. The effects of uncertainty in the underlying model parameters and assumptions are also analyzed, along with the effect of system boundary selection on the assessment outcome. Analysis and testing of the method shows that it can be used as a valuable tool for sustainable process development.
Broader contextThe successful development and industrial implementation of energy and resource efficient, environment friendly, safe and affordable chemical conversion processes is essential for meeting sustainable development goals. However, one of the critical challenges to engage in sustainable process design is the lack of information and availability in a format which can be used by chemists and engineers. Taking steps to meet this challenge, this work presents a methodological tool for early stage multi-criteria assessment of chemical processes. It can be used to evaluate and shape key process development decisions, especially for novel production of renewable fuels and bulk chemicals. |
Introduction
Sustainability is a key challenge for the twenty-first century. Over the past couple of centuries, we have significantly improved our standard of living through increased use of fossil resources. However, our reliance on fossil resources poses critical questions in view of finite resources and environmental impacts. These concerns become even more crucial in the wake of increasingly resource-intensive consumption patterns across the world and have to be balanced against the growing needs of the world population. It is hence imperative to strike a balance between our economic, environmental and societal interests to achieve sustainability.In recent years, an increasing awareness of sustainability issues has led to an impetus for efficiency improvement, hazard minimization and utilization of renewable resources such as biomass. As we develop novel chemical conversions, it is important to analyze these processes within a broader economic, environmental and social context. Such an assessment helps us to identify promising alternatives and channel capital accordingly. The flexibility of early stage process development offers unique opportunities for chemists and engineers to use this assessment and make new pathways inherently sustainable.
A critical challenge while performing an early stage assessment is to work with the limited information available. Green chemistry principles laid down by P. Anastas1 have pioneered sustainability thinking in process development. Although useful, these principles are qualitative in nature and fail to consider trade-offs between the economic feasibility, environmental impacts, risks and benefits associated with the chemical process. There have been other quantitative and qualitative assessment techniques based on specific product and process attributes, such as E-factor,2 GME,3 EcoScale4 and ProSuite.5 More comprehensive methods such as BASF eco-efficiency6 and the Sustainability Consortium Open IO7 rely primarily on data from existing processes or rigorous process and supply chain modeling efforts. The comprehensive methods incorporate features such as techno-economic analysis, environmental and social life cycle assessment, and so forth. Most of these methods are either qualitative and very broad (based on brand image or final product characteristics) or extremely information intensive, which demands significant investment of time and resources. Hence there is a need for a tool that provides a rather quick but informative assessment that can aid in key decision-making at the laboratory stage of a process. For such an assessment, it is important to utilize as much quantitative and qualitative information as is available at an early stage in process development. The work by H. Sugiyama et al.8 represents an important step in this direction. His approach takes into consideration factors such as raw material costs, environmental impacts and hazards and is primarily targeted toward petrochemical processes. In this paper we continue in the direction of H. Sugiyama's work. We modify his approach by incorporating more practical aspects and propose a comparative assessment method for chemical processes at the laboratory stage. Fig. 1 shows the stage in the process development pipeline at which the proposed methodology could be applied. In its current form it is primarily targeted at processes for fuels and bulk chemicals. However, the flexibility of the proposed method could enable additional applications with some minor modifications.
 | ||
| Fig. 1 Process development pipeline and methodology application. | ||
The proposed assessment incorporates basic reaction mass balance information along with data such as raw material prices, greenhouse gas (GHG) emissions and qualitative indicators. This information is integrated by means of weighing factors. In this article we use the method to analyze a biobased process and a comparable fossil-based process. This comparison gives us an important indication of the potential benefits that a proposed new process can offer over a conventional process in terms of sustainability. We also assess the robustness of this outcome in light of uncertainties in the input information.
This method has been developed and applied within the CatchBio program in the Netherlands, which focuses on the development of catalytic processes for conversion of biomass to fuels and chemicals. In this paper we apply this method to a catalytic process for the production of but-1,3-diene from ethanol, which is being developed within the CatchBio program. This process is compared with the dominant conventional method for production of but-1,3-diene from naphtha in a steam cracker. Using the results of this assessment, we analyze the plausibility of the results and explore various details regarding application of the methodology. This method has already been tested for approximately a dozen different processes and the results will be published in the near future.
Methodology description
The method evaluates an innovative new chemical process and a comparable conventional process based on selected parameters that are used as proxies for economic feasibility, environmental impact, human health, and risks and opportunities. This method combines quantitative information about the raw materials and the process with qualitative indicators that reflect the sustainability of the process. The system considered by the assessment method includes the reaction and a separation process that is assumed to be ideal due to the lack of real process data (see Fig. 2). Fig. 2 shows the level of process detail, where S represents the mass flow of various streams. S1 is the mixed input stream while S5 and S6 represent the product and co-product streams. S4 is the recycle stream and S7 is the waste stream. For this analysis, the parameters that contribute to the final score are as follows: | ||
| Fig. 2 Scope and level of detail. | ||
1. Economic constraint.
2. Environmental impact of raw materials.
3. Process costs and environmental impact.
4. EHS index.
5. Risk aspects.
This method uses basic reaction data in conjunction with other information such as the physical and chemical properties of the chemicals, prices, the cumulative energy demand (CED), greenhouse gas (GHG) emissions, market availability and so forth. The first parameter, economic constraint, provides information about the raw material costs relative to the market value of the products. The second parameter combines proxies for the environmental impacts associated with the raw material consumption for the process. While the first two parameters concern raw material requirements, the third parameter represents an indication of the expected costs and environmental impacts associated with the processing of raw materials into final products. The fourth parameter provides information about the hazards associated with the process and can help in the development of inherently safer chemical processes. The final indicator incorporates information about the external market risks and potential technical aspects associated with the process. The first four mid-point parameters are based on the work of H. Sugiyama et al.8 and have been modified for our assessment method. The fifth parameter is an addition to the basic framework proposed by H. Sugiyama. Based on the input from these five parameters, this method enables analyses of a conversion process in terms of its raw material costs and environmental impacts, processing costs, impacts and hazards, and risk aspects. In this assessment scheme, lower values are desirable for each parameter. Fig. 3 provides an overview of the proposed methodology. The following sections explain each of these parameters in detail.
 | ||
| Fig. 3 Overview of the assessment methodology. | ||
Parameters
Economic constraint (EC)
Economic feasibility is critical for the practical implementation and economic sustainability of a chemical process. It is essential that the market price of a product covers the raw material costs and leaves room for processing costs. Economic constraint as defined here represents the raw material costs as a fraction of the value of the products and co-products. This parameter, which is based on quantitative information, is a function of the market prices of the products and co-products, raw material prices and practical yields. The yields are based on complete conversion of raw materials assuming recycle. It is calculated as a ratio of the economic value (market price × mass flow) of raw material inputs to the combined economic value of the products and co-products. The mathematical formulation can be described as follows: | (1) |
For economic constraint, a lower ratio (<1) indicates a higher opportunity in the form of lower feedstock costs relative to the market value of the products. A ratio higher than 1 indicates that the market value of the products and co-products does not cover the raw materials costs. A process with a lower ratio allows more room to accommodate other capital and processing costs.
In this method, the cost of a heterogeneous catalyst is assumed based on catalyst specifications provided by J. P. Lange.9 Based on this reference, it has been assumed that catalyst consumption is below 1 kg catalyst per ton of product, above which catalyst costs can be critical for process feasibility. For homogenous catalysts, if the data indicate that the catalyst is lost through side reactions or with the product, then that is accordingly taken into account. Based on further catalyst studies, more accurate information about the consumption of catalysts can be incorporated.
Environmental impact (EI) of raw materials
This parameter represents the environmental impacts of the raw materials required for the production of a unit mass of product. H. Sugiyama proposes the cumulative energy demand (CED) of all raw materials as an indicator of this impact. The raw material CED represents the total energy requirements from the cradle to the relevant system boundary. In the context of this assessment this system boundary is the inlet factory gate (i.e., the gate to which raw materials are delivered). It represents the total of renewable and fossil energy inputs along with the feedstock energy content. The CED can be a good representative first indicator for a wide range of environmental impacts.10 In this assessment method, we have also included (with weight equal to the CED) the GHG (eq. CO2) emissions associated with all the raw materials. GHG emissions function as an indicator of non-renewable resource use and climate change, which is an increasingly important long-term sustainability issue.11 Only the fossil GHG emissions have been taken into account, thereby also including fossil carbon embedded in the product, i.e. following a cradle-to-grave approach. This choice represents the conservative assumption that the embedded carbon will be released at a later point in time, through utilization in the case of fuels and either waste incineration or the action of micro-organisms in the case of chemicals. The reasoning is that fossil-based carbon will only be recycled after a long time span of millions of years while contributing to global warming and depleted useful carbon resources in the meantime. Biobased carbon, on the contrary, is recycled rather quickly (on a perennial or biennial basis) and causes a significantly lower global warming effect if it is sustainably harvested and converted. The global warming potential is estimated based on a 100 years timeframe using the IPCC 2007 GWP 100 method.12Economic allocation is used to distribute process impacts over all the products and co-products. Allocation enables a comparison on the basis of one unit of main product, which in essence is the functional unit for the assessment. Given the nature of this calculation, the assessment can be applied to any product from the process, regardless of its mass or economic value. Economic allocation has been used as opposed to mass or energy allocation because it accounts for the fact that the process is being operated primarily for economic reasons. This is because the target of a chemical conversion process is usually to achieve a certain functionality in the product which is reflected in the price of the product. It avoids assigning a substantial share of the overall process impacts to low-value by-products (especially relevant if these are produced in large quantities). The relevant equations for this parameter are as follows:

| (2) |
By analogy with CED, the GHG emissions of the raw materials refer to the system cradle-to-factory gate. However, contrary to CED, the cradle-to-factory gate GHG emissions do not include the portion originating from the feedstock. Hence, no subtraction is required, i.e. the raw material GHG emissions are allocated directly using economic allocation. These allocated GHG emissions and the potential GHG emissions from the fossil carbon embedded in the main product (e.g. petrochemical product) are added, to estimate the raw material based GHG emissions for the main product.
 | (3) |
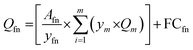 | (4) |
The CED and GHG emissions are good first proxies for environmental impacts, but there are certain limitations for factors such as toxicity. If the required data is available, other factors such as water use and land use can also be incorporated into the method.
Process costs and environmental impact (PCEI)
Given the early stage in process development, it is difficult to obtain quantitative information regarding the costs and environmental impacts involved in conversion of raw materials to products and subsequent downstream processing. Hence this parameter serves as a proxy to give an indication of costs and impacts based on quantitative data inherent to the reaction and products. This index builds upon the energy loss index (ELI) suggested by H. Sugiyama et al.8 and is based on the notion that energy loss in the reaction and separation section of the processing sequence can be used as an indicator for the expected costs and environmental impacts.13The PCEI parameter aggregates seven different indicators, the scores for which are based on the data from the reaction. The individual scores vary from 0 to 1 or from −1 to 0, based on the value of the underlying parameter. The description for the first five parameters follows from H. Sugiyama et al.8 The last two indicators are our proposed additions to the ELI, due to their relevance for new processes (esp. biobased) and in line with other modifications to the method.
 | ||
| Scheme 1 | ||
 | ||
| Scheme 2 | ||
 | ||
| Scheme 3 | ||
 | ||
| Scheme 4 | ||
 | ||
| Scheme 5 | ||
 | ||
| Scheme 6 | ||
 | ||
| Scheme 7 | ||
As a default, equal weights are assigned to each of these indicators contributing to the ELI. The scores of all the indicators are added up to derive the ELI of the process. For processes with multiple reaction steps, a separate ELI is calculated for each reaction and separation step, and the scores of all steps are added to arrive at a single ELI for the whole process.
The use of the mass loss index might seem to penalize low conversions without consideration of selectivity. However, low conversions inevitably lead to additional processing which needs to be considered. Other parameters in the method, such as the EC and the EI, take into account the yield of the product based on complete conversions and selectivity, thus justifying the use of the MLI.
Depending on the availability of data, in addition to the above indicators, catalyst performance could also be included. This could be based on either of the catalyst characteristics, such as turnover frequency, weight hourly space velocity, on-stream time and regeneration time. These characteristics of a catalyst can potentially play a crucial role in the capital and operating costs associated with a project. However, further work is required to develop an operational indicator for catalyst performance.
As an alternative to reaction enthalpy, exergy change in the reaction can also be a useful indicator regarding the energy use and the impacts of processing. The challenge with its use as an indicator is that the calculation involves more steps and certain assumptions have to be made regarding the process heat flows. This can lead to an increase in the difficulty of calculation for this indicator. Hence, considering ease of implementation, reaction enthalpy is used instead of exergy change.
The total energy loss in the process [LHV(feed+fuel) − LHV(product)] can also provide an indication of the capital costs.15 This has not been included because the fuel input for the process is not yet known at the laboratory stage.
EHS index (EHSI)
Hazards are an integral part of chemical processing. It is essential to develop inherently safer chemical processes to minimize hazards and try to prevent incidents such as the Bhopal tragedy.16 Inherently safe processes allow for the reduction of hazard control costs. This index proposed by H. Sugiyama17 and based on G. Koller et al.18 considers the safety, health and environmental (ecological toxicity) aspects of a chemical process and is suitable for an early stage assessment.19 The individual categories and contributing indicators that are aggregated to the EHS index are shown below. The weights for the environment, health and safety categories are 0.4, 0.2 and 0.4, respectively. The calculation of this index is based on the indicator value for each chemical present within the process. Refer to the ESI† for this article and H. Sugiyama8,17 for a detailed explanation of the calculation.(1) Environment (E) (0.4).
∘Persistency (half-life in water).
∘Air hazard (index value of chronic toxicity).
∘Water hazard (L(E)C50 aquatic, R-codes).
∘Solid waste (based on substance class).
(2) Health (H) (0.2).
∘Irritation (EU-class, R-codes, LD50dermal).
∘Chronic toxicity (EU-class, GK, R-codes).
(3) Safety (S) (0.4).
∘Mobility (partial pressure, boiling point).
∘Fire/explosion (flash point, R-codes).
∘Reaction/decomposition (NFPA reactivity, R-codes).
∘Acute toxicity (IDLH, EU-class, GK, R-codes).
The property parameters and hazard classifications of each chemical compound are taken into account to assign index values to each of the parameters. The weights are assigned in such a way that each category within environment, health and safety has equal importance. As originally proposed, the hazards in a process are calculated on the basis of mass flows and indicator values for the chemicals present in the system. In the case of multiple products, we modify the approach suggested by H. Sugiyama by implementing economic allocation to distribute the burden of process hazards over the main product and co-products. Consequently, in the calculation of the category values, the mass flows represented in Fig. 2 should be used instead of the ones used by H. Sugiyama.
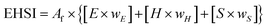 | (5) |
In the case of a process with multiple reaction steps, the methodology to determine the hazard potential can be applied in two different ways: the first approach is to apply the methodology separately for each of the process steps. This results in a higher value for the EHS index because the hazard potential of the intermediate product is considered twice – once as the output from one conversion step and once more as the input to the subsequent step. This seems an adequate approach for a non-integrated facility requiring separate storage, transportation and handling of intermediate raw materials and products. In contrast, twofold consideration of the hazard potential seems inadequate in the case of an integrated facility combining the multiple steps. Thus in the second approach for integrated operation, the EHS methodology is applied jointly over all the conversion steps.
Risk aspects (RA)
This parameter is based on the external economic aspects and technical aspects of the product molecule or reaction pathway, which can play a crucial role in the practical implementation of a new process. It takes into account factors that are not covered explicitly by prices. This parameter has been developed in the context of the CatchBio project framework. The time frame envisioned for the first large-scale implementation of new lab-scale processes is 10–15 years. The indicators have been chosen accordingly and are targeted at processes for commodity chemicals and fuels. The indicators considered are shown below. The respective weights (the numbers after each indicator) are based on expert opinion within the CatchBio project team (socio-economic assessment). Each process is assessed based on scoring statements (qualitative phrases) for each indicator. The overall parameter score is obtained by weighted addition of indicator scores.Feedstock supply risk – 0.25.
Regional feedstock availability – 0.15.
Market risk – 0.25.
Infrastructure (availability) risk – 0.2.
Application-technical aspects – 0.15.
∘ Chemicals: functional groups – 0.5.
∘ Chemicals: retention of raw material functionality – 0.5.
∘ Fuels: high energy content – 0.5.
∘ Fuels: engine compatibility – 0.5.
Inherent functional and pathway (application-technical) aspects can play an important role in unwrapping the future potential for the molecule or pathway. These aspects can open up new markets with greater added value or can act as critical potential barriers. Moreover, the sustained availability of feedstock and a larger market will definitely play a major role in the practical implementation of the process. A process compatible with current infrastructure generally implies a lower risk and investment associated with it. Regional feedstock availability represents local growth opportunities and the avoidance of strategic risks that arise from wars or resource protectionism.
The details of the scoring scheme and qualitative phrases are as follows:
0.5: potential for near-term bulk availability. Multiple equivalent or lower-value applications in sight. Feedstock under development.
1.0: conceptual feedstock (needs fundamental development). Potential applications have a higher value than the one proposed.
This indicator takes into account the global feedstock availability. Technically speaking, a bulk of the available feedstock is only “available” if the proposed application is of a higher value than the current application. For a lower-value proposed application, additional feedstock needs to be produced, since the currently available feedstock will not be diverted from a higher-value application. Hence it is important to take into account the value of the proposed application when feedstock availability is considered.
0.5: feedstock available in other parts of the world in free and open markets.
1: feedstock primarily available in regulated markets with limited global market access.
This indicator is used to incorporate feedstock security issues and local growth opportunities.
0.33: existing commodity (e.g., lactic acid).
0.66: near-term bulk chemical/fuel market potential.
1.0: long-term market potential, possibly accelerated by interesting properties.
0.33: new processing plants are required based on known technologies. Also, the existing target product is part of the existing processing and supply chains.
0.66: new processing plants are required based on known technologies. Also, the target product is new and would need new processing and supply chains.
1.0: new greenfield processing plants built with new technologies. Also, the target product is new and would need new processing and supply chains.
Chemicals. Functional groups (defined as the number of same or different functional groups on the hydrocarbon backbone)
0: between 2 and 4 functional groups. Platform molecule. Wider potential applications.
0.5: more than 4 functional groups. Difficult platform molecule to work with, which can narrow down potential applications.
1: one functional group. Limited potential for platform chemical.
Retention of raw material functionality
0: complete functionality is preserved. Fundamentally efficient approach that can offer future improvement potential.
0.5: limited modification of functionality.
1: all functionality stripped off. Lower theoretical improvement potential.
Fuels. Energy density
0: high energy density. Greater than or equivalent to gasoline/diesel (as applicable).
0.5: energy density 80–90% that of gasoline/diesel.
1: energy density below 80% that of gasoline/diesel.
Engine compatibility
0: perfectly compatible. Gasoline/diesel equivalent. No engine modification required for use.
0.5: potential for use in existing engines when mixed with gasoline/diesel.
1: engine modification necessary for use. Will be a critical application barrier.
Normalization and weighting
The parameters considered in this assessment fall into different categories and as such their scores cannot be added together directly. For this reason, the scores for the new process are normalized against the respective scores for the comparable conventional process. The scores are normalized to 1, meaning that each score is divided by the maximum of the two. Thus the process with a higher raw score gets a 1 and the other process gets an accordingly lower score. Table 1 explains this using the economic constraint (EC) score as an example.| New process | Conventional process | |
|---|---|---|
| Raw EC score | B | P |
| Normalized EC score |

|
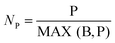
|
The normalized scores for each parameter are added together using their respective weighting factors. The proposed weights for the five different parameters are as shown in Table 2.
| Parameter | Weight |
|---|---|
| Economic constraint (EC) | 0.3 |
| Process costs and environmental impact (PCEI) | 0.2 |
| Environmental impact of raw materials (EI) | 0.2 |
| EHS index (EHSI) | 0.2 |
| Risk aspects (RA) | 0.1 |
The reasoning leading to these weights is as follows:
In today's market-economy-driven and competitive world, a process will not be implemented on a commercial scale unless it is economically feasible. Therefore, economic constraint is assigned a relatively high weight. The next parameter, the process costs and environmental impact, can play a significant role in the economic feasibility of the process while also contributing to the environmental life-cycle impacts of the process. We assume that the PCEI parameter contributes equally to the cost and the environmental impact parameters (i.e., 0.1 each), effectively increasing the weight of cost-related aspects to 0.4.‡ If the process makes economic sense, then – with the goal of long-term sustainability and the minimization of environmental impact – life-cycle environmental impacts have to be taken into account. Hence the environmental impact of raw materials has an effective overall weight of 0.3,§ which is lower than the weight for costs. The EHS index represents relatively short-term or immediate hazards associated with the process. Even though these are extremely important, especially in a social context, these hazards can be controlled, albeit at an increased cost. Hence the EHS index has a relatively lower weight of 0.2 and this argument also supports the higher weight for costs. The risk aspects can potentially be crucial; however, the uncertainty in quantifying the effects of these parameters is quite high. Hence this factor has the lowest weight of 0.1 based on the uncertainty coupled with the lack of definite information regarding the importance of these factors at an early stage of development.
Total score and index ratio
Following the multi-criteria approach, a total score is estimated based on the normalized scores for the process for each parameter and the corresponding weighting factors. The following equations detail the calculation. | (6) |
 | (7) |
 | (8) |
The index ratio IB,P, calculated using eqn (8), is a ratio of the total score for the new process to that for the conventional process. This is the final outcome for the model and gives an indication of the potential benefits associated with the proposed novel process. As such, a lower index ratio (<1) indicates that the new process can provide certain benefits compared with the conventional process.
Uncertainty and sensitivity analysis
The index ratio that we calculate is based on a model with a variety of data inputs and assumptions. There is always uncertainty associated with the data inputs (e.g., the yields can change in practice, and market prices change all the time). Subjectivity is involved, especially in the weighting, and hence different people in diverse situations can have different opinions about them and may change them accordingly. In light of these uncertainties it is important to analyze the variation in outcome and its robustness. This is a crucial step in the utilization of any model outcome for decision-making purposes. For this method, we analyze the effect of these uncertainties using the Monte Carlo analysis technique. This provides us with the distribution of results for a wide range of possible scenarios. A quick analysis of this distribution can give us a good indication of the robustness of the outcome and its usefulness for decision-making. We consider the effect of variations in factors such as prices, yields, the CED and GHG emissions. For this purpose we take into account historical variations in prices and price correlations for key raw materials and products. For information about uncertainties in the CED and GHG emissions, alternative datasets and values from the Ecoinvent database12 have been used. The software @RISK20 has been used to examine the effect of random variations in these inputs on the index ratio. Given the semi-quantitative proxy nature of other parameters (e.g., PCEI) it is difficult to objectively include the uncertainty in such parameters for Monte Carlo analysis. Hence only the aforementioned parameters and inputs are taken into account for the uncertainty assessment.In a multi-criteria assessment such as the one conducted here, the use of weights for different categories can have a profound effect on the outcome and the conclusions that are subsequently drawn. Thus we analyze the effect of variations in the weighting factors for the five different parameters on the outcome. To this end, 1000 different randomly generated weighting sets within the ranges specified in Table 3 are used. Given the selection and nature of the parameters (e.g., cost aspect covered by two parameters) under consideration, these ranges enable us to generate plausible as well as varied weighting sets. These random weighting sets, in which the sum of weights is always ‘1’, are generated using an Excel based algorithm that we developed specifically for this purpose. While generating these weighting sets, the environmental impact parameter is broken down into the CED and the GHG emissions. Separately varying the weights for the CED and GHG emissions enables us to incorporate viewpoints that place higher importance on some environmental impacts than others.
| Parameter | Default weights | Weight ranges | |
|---|---|---|---|
| Min | Max | ||
| Economic constraint (EC) | 0.3 | 0.25 | 0.60 |
| Process costs and environmental impact (PCEI) | 0.2 | 0.15 | 0.35 |
| Cumulative energy demand (CED) | 0.1 | 0.05 | 0.30 |
| Greenhouse gas emissions (GHG) | 0.1 | 0.05 | 0.30 |
| EHS index (EHSI) | 0.2 | 0.05 | 0.30 |
| Risk aspects (RA) | 0.1 | 0.05 | 0.25 |
In future, a scheme of weights based on parameter or specific indicator scores can also be envisioned to better incorporate and reflect the assessment context and different viewpoints (e.g. the importance of cumulative energy demand compared to GHG emissions within the parameter EI).
Apart from uncertainty analysis, to incorporate the focus on yields, the sensitivity of the outcome to different yield scenarios is also considered. This gives an indication of the change in outcome with changes in yields of the main product under consideration. Two scenarios, one positing a 20% decrease in yields and one positing theoretical yields, are considered in this assessment.
Laboratory decision-making
The primary goal of this work is to provide an assessment tool for processes that are in an early development stage. It should be used carefully so as to avoid stifling innovation. Rather, it should be used to guide innovation toward sustainability. At an early stage it can be used to pinpoint bottlenecks and set research targets in process development. It can aid in analyzing potential alternatives being considered in the laboratory, within a broader context. As an example, the tool can provide a basis to evaluate the costs and benefits of using a certain toxic solvent that leads to higher yields against those of using a greener solvent with lower yields and potentially useful by-products. Thus, using such an assessment, key decisions that are made as the process is being developed can result in a sustainable process.Results and discussion
Comparison of bioethanol and naphtha routes for but-1,3-diene production
To assess the methodology and examine the plausibility of the results, it has been applied to a biobased and a petrochemical but-1,3-diene production process. The biobased process under consideration involves the production of but-1,3-diene from bioethanol over heterogeneous chemical catalysts.21,22 This process is compared with its dominant petrochemical counterpart in which but-1,3-diene is produced by steam cracking of naphtha.23 Further details for the processes can be found in the ESI†. The method is simultaneously applied to both processes and the individual parameter scores are normalized. The results for each of these parameters are examined in detail in the following sections.Parameter assessment results
Fig. 4 shows a comparison of the parameter “Economic constraint” for the two processes. It indicates feedstock costs for the process as a fraction of the market value of the products and co-products. The result is based on European market prices24 for bioethanol (0.78 € kg−1), naphtha (0.63 € kg−1), ethylene (0.98 € kg−1) and but-1,3-diene (1.32 € kg−1) in November 2010 and average 2010 prices24 for other chemicals. The naphtha-based process offers greater economic leeway for processing, compared with the bioethanol-based process. However, it is important to note that the market prices change continuously based on supply and demand. A process developer needs to realize that an economic constraint above 1 does not necessarily mean that the process is not worth pursuing. An uncertainty and sensitivity analysis in conjunction with an evaluation of the market outlook should be used for decision-making based on this information. For example, if, even after considering theoretical yields and optimistic market scenarios, the economic constraint is above 1.5–2, that is a strong indication for exploring alternatives. In this particular case of but-1,3-diene production processes, there have been wide variations in the price of but-1,3-diene over time.24 On the supply side, greater steam-cracking capacity is expected to be put into operation in the Middle East. This capacity will be increasingly based on lighter feedstocks (ethane, propane). This could decrease co-production of C4s and thus but-1,3-diene. On the other hand, there is an increasing demand for but-1,3-diene from China, India and other growing markets. With this market outlook, one could expect favorable economic opportunities for an bioethanol-based but-1,3-diene process. | ||
| Fig. 4 Economic constraint comparison for but-1,3-diene from bioethanol and naphtha. +The scores presented in this figure have not been normalized. | ||
Fig. 5 shows the comparison of the CED and GHG emissions associated with the bioethanol- and naphtha-based but-1,3-diene production processes. The CED and GHG emission data for raw materials is obtained from the Ecoinvent database12 and EU directive 2009/28/EC.25 Bioethanol-based but-1,3-diene has a higher overall CED compared with naphtha-based but-1,3-diene. This is primarily due to the fact that the CED includes both renewable and non-renewable energy. The naphtha process has undergone extensive process and supply chain optimization in the past decades, thus making it more efficient. In comparison, the bioethanol process is relatively new and involves energy inputs to agriculture and the harvesting of crops in addition to chemical conversion. It is also more process-intensive to make a product from solid biomass compared with liquid crude oil. In a way, this higher CED also supports the opposite outcome observed for the PCEI (see Fig. 6), since the energy inputs included in the CED occur outside of the system boundary of the PCEI. It is important to note that the allocation approach also plays a role in the final CED value for but-1,3-diene.
 | ||
| Fig. 5 CED and GHG emissions for but-1,3-diene from ethanol and naphtha route. | ||
 | ||
| Fig. 6 PCEI scores for bioethanol- and naphtha-based but-1,3-diene processes. | ||
In contrast to the CED, the GHG emissions are higher in the case of naphtha-based but-1,3-diene. This deviation from the CED trend is observed because the emissions associated with the naphtha-based route include future emissions from fossil carbon embedded in the but-1,3-diene product, which will eventually be released into the atmosphere as CO2. The GHG emission value of boiethanol is based on the EU directive 2009/28/EC25 for biofuels. The value used is based on a mandated 35% reduction in GHG emissions of bioethanol compared with gasoline. In this directive, the current 35% reduction requirement is set to be reduced further to 60% by 2018. Thus further reductions in bioethanol GHG emissions can be expected in the coming years.
Fig. 6 shows a comparison of potential process costs and environmental impacts for but-1,3-diene production based on the energy loss index and the various contributing factors. In this case, both processes are based on only one reaction and a subsequent separation step. The scores compared in Fig. 6 are raw scores for each process and have not been normalized. The bioethanol-based process involves one reaction step and three co-products. This makes it a relatively simple conversion process with lower separation requirements. The naphtha-based process involves a large number of products (>9), some with fairly close boiling points, which need to be separated. On a mass basis, but-1,3-diene is only 5% of the output stream from the steam cracker. In general, steam cracking is also a strongly endothermic reaction, thus demanding large additional energy inputs. In line with expectations, the model indicates that the naphtha-based process needs relatively more intensive processing compared with the bioethanol-based process. Thus relatively lower processing costs and environmental impacts can be expected in the case of a bioethanol-based but-1,3-diene process.
Fig. 7 shows the comparison of the EHS index (EHSI), which is based on the hazard scores of the processes as allocated to the but-1,3-diene product. It is evident that the naphtha-based but-1,3-diene process carries a moderately higher hazard compared with the bioethanol-based but-1,3-diene process. The hazard index is based on the specific mass flows of the chemicals per unit of product within the process. Both processes lead to one metric ton of but-1,3-diene, which carries an identical hazard potential in both cases. The difference in scores shown in Fig. 7 therefore originates from the hazard potential of the respective inputs and other co-products. The more hazardous characteristics of naphtha and steam-cracking co-products compared with ethanol explain the higher EHS index.
 | ||
| Fig. 7 Comparison of process hazards for bioethanol- and naphtha-based but-1,3-diene. | ||
In this method, we also assess certain risk aspects associated with a conversion process. Fig. 8 shows a comparison of this parameter for the two routes of but-1,3-diene production. In Fig. 8, not all the indicators are displayed on the bar chart since some indicators have a score of 0 for the processes being compared. Given the timeframe considered, both feedstocks can be expected to be widely available in large quantities. The market value of but-1,3-diene is higher than the value of bioethanol for fuel use. Thus there is a good probability that bioethanol will be available for processing to but-1,3-diene through an economically feasible process. This indicates a low feedstock supply risk (therefore zero score for both routes).
 | ||
| Fig. 8 Risk aspects index comparison. | ||
But-1,3-diene has a well-established commodity-scale market that is expected to grow further. Thus we expect a low market risk. In the case of the bioethanol-based process, new infrastructure and logistics will need to be developed for processing, which entails additional risks. In comparison, the addition of new capacity based on existing naphtha-based technology has considerably lower risks.
This particular analysis has been considered from the perspective of implementation of the process in Europe. In the case of naphtha, large-scale availability in the EU will be dependent upon imports from countries outside the EU, which would more or less be classified under free markets. However, bioethanol production in the EU is increasing, which will enable the benefits of regional feedstock availability for but-1,3-diene production. In this case, since the target molecule is the same, the technical aspects associated are similar.
Overall, based on the weighting factors, the bioethanol-based process has a comparatively lower score for this parameter. For the given timeframe and context, this parameter gives a good indication of the risk aspects associated with the biobased process. For different contexts, such an indicator or the respective weights can be modified accordingly and used to incorporate external qualitative information in the assessment scheme.
Integrated score
Integrating the scores for each parameter, Fig. 9 shows the overall comparison of bioethanol- and naphtha-based but-1,3-diene processes using the baseline weights which are indicated in parentheses. As lower scores are better, the figure indicates that the bioethanol-based process has an edge over the petrochemical process. Table 4 shows the raw scores for each of the parameters considered. For a bioethanol-based process, one can expect comparatively lower processing costs, process hazards and marginally lower risks. However, the bioethanol-based process has a comparatively higher economic constraint and a similar environmental impact of raw materials. The total score of the bioethanol-based route is 0.81 compared with 0.90 for the naphtha route. Thus the index ratio for the bioethanol-based process is 0.90. This indicates that the bioethanol-based process may be beneficial. Apart from its use for evaluating and improving the new process, the index ratio can also be used to rank different process options. If one were to evaluate the potential benefits in terms of magnitude of contribution to the society, then in addition to the beneficial index ratio, the market size of the product could also be explicitly considered. | ||
| Fig. 9 Bioethanol- and naphtha-based but-1,3-diene process comparison. | ||
| Parametersa | Bioethanol-based | Naphtha-based |
|---|---|---|
| a Lower values are better for the respective processes. b Cumulative energy demand (MJ kg−1 but-1,3-diene): 118.96 (bioethanol); 61.17 (naphtha). GHG emissions (kgCO2 eq. kg−1 but-1,3-diene): 2.45 (bioethanol); 3.98 (naphtha). | ||
| Economic constraint (index) | 1.00 | 0.83 |
| Environmental impact of raw materials (normalized index)b | 0.81 | 0.76 |
| Process cost and environmental impact (index) | 1.93 | 3.60 |
| EHS hazard potential (index) | 1.95 | 2.67 |
| Risk aspects (index) | 0.14 | 0.15 |
Uncertainty and sensitivity analysis
The index ratio gives a good first indication of the sustainability of a biobased process option. To evaluate the robustness of this result and aid in decision-making, an uncertainty and sensitivity analysis has been carried out. A 20% decrease in the yield from ethanol would lead to an index ratio of 0.91. In the case of theoretical yields of but-1,3-diene from ethanol, the resulting index ratio is 0.89. The relatively minor change in the index ratio can be attributed to the fact that the combined value of all the products and co-products from the reaction is considered. Thus a 20% yield decrease for but-1,3-diene production results in a corresponding increase in production of co-products. It is important to note that this change depends on the value of the co-products. If the co-products produced are of low economic value, then a change in yields can lead to significant variations in the index ratio.Fig. 10 and Table 5 show the results of the Monte Carlo analysis based on the uncertainty in the estimated environmental impact and economic feasibility. The uncertainty in parameters such as yields, the CED and GHG emissions has been incorporated. In the case of economic data, the uncertainty in prices for bioethanol, naphtha, ethene, propene and but-1,3-diene has been used. Quarterly prices from January 2007 to November 2010 have been taken into account.24,26 This range incorporates the wide variation in chemical and fuel prices that was experienced during this time frame. The results indicate that in terms of the index ratio, the bioethanol-based process can be expected to provide benefits in 90% of the scenarios. These statistics support the outcome, which indicates that bioethanol-based but-1,3-diene can provide certain benefits compared with the naphtha-based process.
 | ||
Fig. 10 Histogram of Monte Carlo simulation results for base-case weighting set (N = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). 000). | ||
| Parameter | Value |
|---|---|
| Mean | 0.87 |
| Standard deviation | 0.10 |
| Minimum | 0.60 |
| Maximum | 1.46 |
| Kurtosis | 4.3 |
However, the uncertainty analysis reported in Fig. 10 is based on a particular weighting set, which represents a viewpoint in a general context. As an example, in some regions of the world, the risk aspects might carry a high weight. Fig. 11 shows the distribution of the index ratio for a wide range of randomly selected different weighting sets, within specified ranges. These index ratios are estimated for the default set of parameter values. The mean value of this distribution is 0.92, while the standard deviation is 0.05. This reaffirms the validity of the outcome over a wide range of different viewpoints.
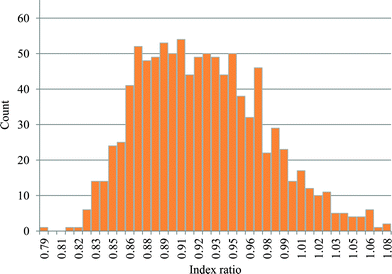 | ||
| Fig. 11 Histogram of Monte Carlo simulation results with variation in weighting sets and default parameter set for bioethanol-naphtha comparison (N = 1000). | ||
System boundary discussion
For this assessment method one could use different system boundaries, which involves consideration regarding which raw material to start with and where it lies along the value chain. To assess the effect of a change in system boundaries on the model's outcome, we consider the biobased but-1,3-diene production process. The two respective system alternatives have been shown in Fig. 12 and 13. In both figures, solid dark arrows represent quantitative information based on the market data or detailed modeling efforts. The hatched arrows represent qualitative information based on indices, which is used in the absence of quantitative information. The width of an arrow represents the weight assigned to that particular aspect. The bubbles represent information that is implicitly incorporated in the information carried by the arrows and the model in general. We combine these information flows using weights into a total score. | ||
| Fig. 12 Information flows using a smaller system boundary. | ||
 | ||
| Fig. 13 Information flows using an extended system boundary. | ||
The results presented earlier for the ethanol-to-but-1,3-diene process (Fig. 9) are represented by the system shown in Fig. 12. Alternatively, instead of using ethanol as our starting point, we could start with glucose. This second alternative is represented in Fig. 13. In this case, we analyze the glucose-to-ethanol and the ethanol-to-but-1,3-diene conversion steps. The integrated scores for the comparative assessment of glucose-based and naphtha-based but-1,3-diene are shown in Fig. 14. The total scores in this case are 0.82 and 0.95, respectively, for the glucose- and naphtha-based processes. Thus the index ratio works out to 0.87. Please refer to the ESI† for an additional explanation about the interaction and interdependence of different parameters in reference to the system boundary.
 | ||
| Fig. 14 Glucose- and naphtha-based but-1,3-diene process comparison. | ||
The key question here is how to select the system boundary. Life-cycle assessment follows the approach of extending the system back to the cradle in order to include the environmental impacts of the entire process chain; a more complete analysis ensures more accurate results. Based on this example, one may consider the approach in Fig. 13 with an extended system boundary to be more accurate than the one in Fig. 12. However, the opposite is valid for this assessment because we utilize a mix of background and foreground information. The approach for this method is based on the assumption that the price, the CED and the GHG emissions of raw materials carry quantitative information regarding the costs, hazards and environmental impacts involved in the production of the raw materials. For the extended system represented by Fig. 13, quantitative and rather accurate information is obtained for the glucose raw material. This information is then complemented with qualitative and semi-quantitative information (PCEI, EHSI) for the glucose-to-ethanol and ethanol-to-but-1,3-diene conversion steps. In the case of the system represented by Fig. 12, quantitative and again relatively accurate information is obtained for the ethanol raw material. This information is then complemented with qualitative and semi-quantitative information for only the ethanol-to-but-1,3-diene conversion step. Hence in the case of a smaller system boundary, the assessment relies more on external quantitative information and less on qualitative and semi-quantitative information about the process.
As an example, to get an indication of the energy demands of but-1,3-diene production from ethanol, both the CED value for ethanol and the energy loss index (ELI) are used. The latter can be seen as a proxy (qualitative information) for the energy requirements related to the conversion of ethanol to but-1,3-diene. The combination of this information with the CED of ethanol can be seen as a proxy for the CED of but-1,3-diene. The CED for ethanol represents definite information based on detailed modeling efforts and data. This information is complemented with indicative information using the energy loss index for the process cost and environmental impact to get an indication of the CED of but-1,3-diene without detailed modeling. In the case of an extended system boundary, however, in addition to quantitative information on the CED of glucose, the outcome relies on two sets of proxies (qualitative information): first for the glucose-to-ethanol and then for the subsequent ethanol-to-but-1,3-diene conversion step. Thus a smaller system boundary ensures that the outcome from the model is based on higher-quality quantitative information. Hence a system boundary representing exclusively the conversion of ethanol to but-1,3-diene (Fig. 12) should provide the most accurate evaluation. However, in the case of a category such as EHS hazards, there is a tradeoff involved in having a smaller system boundary. To some extent, it can be assumed that hazard costs are estimated and priced into the product price through insurance and investments into hazard control mechanisms. However, the internalization of hazard costs into the price of the product depends on local governmental laws and the regulatory framework in the region where the product is produced. If there is only limited legal enforcement in countries representing a substantial part of global production, this could explain lower production costs and hence lower prices; in this case, prices would not properly reflect good practice in hazard control. It also relies on the very definition of hazards, which can vary across regions. Some aspects might not be viewed as hazards in some regions, while they might be classified as hazards in others. In such a scenario, a smaller system boundary can be less desirable because it increases the reliance of the outcome on externally estimated hazards built into prices rather than on concrete hazard indices estimated within the model. Nevertheless, given the uncertainty in hazard classification and estimation, combined with the weight for each hazard category, we believe the outcome from the model would be more plausible in the case of a smaller system boundary.
Conclusion
The proposed method builds upon existing methodologies and combines aspects of techno-economic analysis, life-cycle assessment and green chemistry. Results from the model give a good preliminary indication regarding the sustainability of a new process compared with a similar conventional process. The results from a preliminary assessment seem plausible and fairly in line with reality and practical expectations. The base-case assessment of but-1,3-diene production gives an indication of the benefits of a biobased process over a petroleum process. Sensitivity and uncertainty analyses indicate the robustness of the model and aid in decision-making. However, it is imperative that the prices used for assessment fall within a similar time range for all the chemicals and that they are based on balanced markets (i.e., no particular shortage or excess of any of the core chemicals).Expansion of the system boundary may not seem to have a profound effect on the outcome from the model for the analyzed case. However, given the structure of the model and the underlying assumptions, we consider the approach based on a smaller system boundary to be more accurate. In making this choice some acceptable tradeoffs have to be made, as in the case of hazard estimation.
The use of allocation enables a fair comparison of the costs and impacts associated with a product from a particular process. The role of economic considerations as the driver for decisions about the process design and its operation justifies the use of an economic allocation methodology. Studying the contribution of specific inputs (e.g., chemical hazard indices) within the model can aid in highlighting opportunities for modifications in the process to enhance efficiency and sustainability.
In any model-based assessment, the quality of the outcome is dependent on the quality of the data input. This model requires a multitude of preliminary data inputs in the form of practical yields, prices, life-cycle data, and the physio-chemical and toxicological properties of chemicals. It is important to ensure that good quality data are efficiently collected to enable a quick and informative assessment of various new conversion processes. In cases where exact data are not available for a process or chemical, these should be substituted with data based on reasonable assumptions that are clearly explained. Based on the availability of data, the model can be modified to include additional information (e.g., land use, water use) regarding the sustainability of the pathway under consideration.
This method has been applied and tested for a number of processes within the CatchBio program. Assessment of additional processes using this method will be useful in establishing the broad applicability of this assessment method. The results from further assessments using this methodology will also provide an opportunity to fine-tune the qualitative aspects of the scoring methodology. In-depth examination of the model inputs and an assessment of its calculation techniques can be crucial in establishing plausibility of results. In the future it can be worthwhile to further improve the method by developing operational indicators for some aspects such as catalyst costs and performance.
At initial stages of process development, this method provides a good alternative to assessment based on full process design as in the case of techno-economic or life-cycle assessment. Overall, this method forms a basis for a rather quick preliminary assessment of novel chemical processes. It can aid in laboratory decision-making, thus proving useful in guiding innovation towards a sustainable future.
Acknowledgements
This research was supported through the CatchBio project by the SmartMix program of the Dutch government.Notes and references
- P. T. Anastas, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed.
- R. A. Sheldon, Green Chem., 2007, 9, 1273 RSC.
- J. Auge and M. Scherrmann, New J. Chem., 2012, 36, 1091–1098 RSC.
- K. Van Aken, L. Strekowski and L. Patiny, Beilstein J. Org. Chem., 2006, 2, 3 CrossRef.
- D. Rai, M. Harmelink, M. K. Patel, M. Goedkoop, J. Fontes, S. DeMeester, J. DeWulf, P. Sellke, R. Schroeter, L. Talens, G. Villalba and R. Ayers, Overview of Essential Technology Features and Parameters for the Assessment of Emerging Technologies, 227078, PROSUITE, Utrecht, 2010 Search PubMed.
- P. Saling, A. Kicherer, B. Dittrich-Krämer, R. Wittlinger, W. Zombik, I. Schmidt, W. Schrott and S. Schmidt, Int. J. Life Cycle Assess., 2002, 7, 203–218 CrossRef.
- R. W. Cox, OPEN IO, the Sustainability Consortium, 2001 Search PubMed.
- H. Sugiyama, U. Fischer, K. Hungerbühler and M. Hirao, AIChE J., 2008, 54, 1037–1053 CrossRef CAS.
- J. P. Lange, CATTECH, 1999, 3, 51–52 Search PubMed.
- M. A. Huijbregts, S. Hellweg, R. Frischknecht, H. W. Hendriks, K. Hungerbuhler and A. J. Hendriks, Environ. Sci. Technol., 2010, 44, 2189 CrossRef CAS.
- J. Rockström, W. Steffen, K. Noone, Å. Persson, F. S. I. Chapin, E. Lambin, T. M. Lenton, M. Scheffer, C. Folke, H. J. Schellnhuber, B. Nykvist, C. A. de Wit, T. Hughes, S. van der Leeuw, H. Rodhe, S. Sörlin, P. K. Snyder, R. Costanza, U. Svedin, M. Falkenmark, L. Karlberg, R. W. Corell, V. J. Fabry, J. Hansen, B. Walker, D. Liverman, K. Richardson, P. Crutzen and J. Foley, Ecol. Soc., 2009, 14, 32 Search PubMed.
- Pre Consultants, SIMAPRO Version 7.3, 2011 Search PubMed.
- A. A. Bumann, S. Papadokonstantakis, H. Sugiyama, U. Fischer and K. Hungerbühler, Energy, 2010, 35, 2407–2418 CrossRef CAS.
- E. Heinzle, D. Weirich, F. Brogli, V. H. Hoffmann, G. Koller, M. A. Verduyn and K. Hungerbühler, Ind. Eng. Chem. Res., 1998, 37, 3395–3407 CrossRef CAS.
- J. P. Lange, CATTECH, 2001, 5, 82–95 CrossRef CAS.
- I. Eckerman, Master of Public Health – Essay, 2001.
- H. Sugiyama, PhD thesis, ETH Zurich, 2007.
- G. Koller, U. Fischer and K. Hungerbühler, Comput. Chem. Eng., 1999, 23, S63–S66 CrossRef.
- I. K. Adu, H. Sugiyama, U. Fischer and K. Hungerbühler, Process Saf. Environ. Prot., 2008, 86, 77–93 CrossRef CAS.
- Palisade Corporation, @RISK.
- C. Angelici and P. C. A. Bruijnincx, personal communication.
- S. Kvisle, A. Aguero and R. P. A. Sneeden, Appl. Catal., 1988, 43, 117–131 CrossRef CAS.
- M. L. Neelis, M. Patel, D. J. Gielen and K. Blok, Resour., Conserv. Recycl., 2005, 45, 226–250 CrossRef.
- ICIS, Chemical Week, 2007–2010 Search PubMed.
- European Union, Directive 2009/28/EC.
- R. Gelten, S. Kin, L. Nardon, M. van der Bijl, D. van Doren, M. Junginger and G. Jonker, IEA Bioenergy Task 40/EUBIONETIII Country Report for the Netherlands – Update for 2009, NWS-E-2009-52, IEA Bioenergy, Utrecht, 2010 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ee21581k |
| ‡ 0.1 (from the parameter “Process costs and environmental impact”) + 0.3 (from the parameter “Economic constraint”) = 0.4. |
| § 0.1 (from the parameter “Process costs and environmental impact”) + 0.2 (from the parameter “Environmental impact of raw materials”) = 0.3. |
| This journal is © The Royal Society of Chemistry 2012 |
