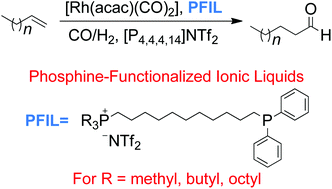
Monodentate phosphine-functionalized phosphonium ionic liquids (PFILs) were employed as ligands for Rh complexes and used in the hydroformylation of higher alkenes. Three PFILs were designed by varying the length of the P-alkyl chain attached to the phosphonium moiety, for alkyl = methyl (1), butyl (2), octyl (3), in order to tune their solubility properties. In all PFILs, the phosphonium unit is linked to a diphenylphosphino functionality by an undecyl linker, with bis(trifluoromethylsulfonyl)imide as counter anion. These PFILs were combined with a Rh(I) precursor, [Rh(acac)(CO)2], to provide a biphasic hydroformylation catalyst for the transformation of 1-octene, 1-decene and 1-dodecene using tetradecyltributylphosphonium bis(trifluoromethylsulfonyl)imide, [P4,4,4,14]NTf2 as a solvent. Good activities and excellent selectivities were obtained for these PFILs-Rh(I) complexes. Variation of the P-alkyl length in the PFIL ligand influenced the stability, catalytic activity and selectivity of the PFIL-stabilized catalyst.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...