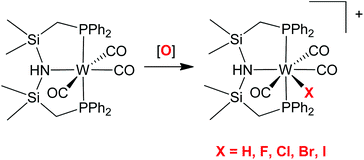
Reaction of the neutral PHNP ligand [HN(SiMe2CH2PPh2)2] with tungsten hexacarbonyl resulted in coordination of PHNP through both phosphorus donor atoms to form the tungsten complex [W(PHNP)(CO)4] (1). Reaction of PHNP with tris(acetonitrile)tricarbonyl tungsten gave both facial and meridional tridentate isomers [W(PHNP)(CO)3] (2-fac and 3-mer). These three d6 tungsten complexes could be interconverted under appropriate conditions. The thermodynamically favored isomer 3 was protonated to form seven-coordinate [W(PHNP)(CO)3H][BF4] (4). A related series of cationic tungsten(II) halide complexes was synthesized, [W(PHNP)(CO)3X]+ (6, X = I; 7, X = Br; 8, X = Cl; 9, X = F), by various routes. All of the tungsten(II) complexes underwent deprotonation at the amine site of the PHNP ligand when triethylamine was added, resulting in neutral seven-coordinate complexes. Variable temperature 1H, 31P{1H}, and 13C{1H} NMR spectroscopy showed fluxional behavior for all the seven-coordinate complexes reported here. Analysis of IR and NMR spectroscopic data showed trends through the series of coordinated halides. Crystal structures of tetracarbonyl 1, meridional tricarbonyl 3, and cationic hydride 4 were determined to confirm the coordination mode of the PHNP ligand.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...