Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealised
Edward R. T.
Tiekink
*
Department of Chemistry, University of Malaya, 50603, Kuala, Lumpur, Malaysia. E-mail: Edward.Tiekink@gmail.com; Fax: 60 3 7967 4193; Tel: 60 3 7967 6775
First published on 17th January 2012
Abstract
Despite being disparaged for their malodorous and toxic demeanour, compounds of selenium, a bio-essential element, and tellurium, offer possibilities as therapeutic agents. Herein, their potential use as drugs, for example, as anti-viral, anti-microbial, anti-inflammatory agents, etc., will be surveyed along with a summary of the established biological functions of selenium. The natural biological functions of tellurium remain to be discovered.
 Edward Tiekink | Edward Tiekink is a graduate of The University of Melbourne where he obtained his Ph.D. (1985) and later a D.Sc. (2006) in Inorganic Chemistry/X-ray Crystallography. He joined the University of Adelaide in 1989 and, after 16 years, decided that it was time to move on and meet new challenges. Edward is now based at the University of Malaya and is establishing research groups in metal-based drugs as well as in his primary scientific passion, crystal engineering. |
Background
Several main group elements have therapeutic roles in contemporary medicine. Prominent examples include the utility of tin(IV) porphyrin compounds (e.g. Purlytin®) to function as agents in photodynamic therapy for the treatment of age-related macular disease and skin cancers,1a the anti-leukaemic activity of arsenic in the form of “As2O3”,1b and the time-proven utility of pentavalent antimony compounds (sodium stibogluconate (Pentostam®) and meglumine antimoniate (Glucantime®)) for the treatment of cutaneous leishmaniasis, a parasitic disease spread by the bite of a sandfly.1c An example of an over-the-counter medicine are bismuth formulations (e.g. bismuth subsalicylate in Pepto-Bismol®) designed to target Helicobacter pylori to relieve the symptoms of peptic and duodenal ulcers, and chronic gastritis.1d Against this background, the medicinal applications of selenium and tellurium compounds are clearly in their infancy. In the following, after a brief overview of the key biological role of selenium, an evaluation of the pharmaceutical potential of selenium and tellurium compounds against different diseases will be presented.The metalloid, tellurium, is named after the Roman earth goddess, Tellus, and its counterpart above it in the Periodic Table, selenium, is, appropriately, if not by chance, named after one of the Greek goddesses of the moon, Selene.2a By contrast to tellurium which does not manifest any known natural biological function,2b selenium is a bio-essential element, identified in the early 1950s as being vital for several organisms, including mammals.2c–e Selenocysteine is the 21st amino acid and is the principle form in which selenium is incorporated in selenoproteins, as reported in 1973.2f,g Selenocysteine is present in the active site of the mammalian anti-oxidant glutathione peroxidase, iodothyronine deiodinase, responsible for the conversion of thyroid hormones, and in thioredoxin reductase, which plays a role in DNA synthesis.2h–m Based on the results of macromolecular crystallography, selenium is known to be present in over 40 different forms (inorganic, organic and synthetic) and is known to interact with both DNA and RNA, as well as proteins, as mentioned above.2n Given the role of “selenium” in various metabolic pathways including DNA synthesis, drug discovery strategies now are evolving to target selenoproteins and H2Se, a key precursor required for the synthesis of selenoproteins.2o
A healthy diet for adults entails 55–70 μg of selenium per day3a and when dietary levels of selenium exceed 1 mg day−1, symptoms of toxicity manifest (i.e. selonosis3b) as depression, fatigue, memory-loss, hair-loss, malodorous breath, etc.3c By contrast, dietary intake below 1 μg kg−1 can lead to selenium deficiency and sickness such as, prominently, Keshan disease and Kashin-Beck disease. Endemic in Keshan County (in Heilongjiang Province in far North-East China) from which the name of the disease was derived, the potentially fatal Keshan disease afflicts the heart muscle (cardiomyopathy) of children and child-bearing women, and is linked with selenium deficient soils in Keshan and in a large swath across China, from the North East to Tibet. While still controversial,3d the lack of nutritional selenium is thought to be correlated with the emergence of a more virulent form of the coxsackie B3 virus. Remediation of the disease can be achieved through dietary supplementation with selenium. An additional widespread syndrome associated with selenium deficiency is Kashin-Beck disease, a chronic disease of the bone and cartilage (osteochondropathy) that afflicts children (5–15 years) leading to pain, stiffness and enlargement of the joints.
While beyond the specific topic of “therapeutic agents”, selenium supplementation (by inorganic salts such as sodium selenite, sodium selenate, and by organic-selenium such as selenomethionine) is also controversial.3c–e Selenium supplementation is proposed because of its anti-oxidant characteristics and has been suggested to reduce the risk of certain types of cancers, arthritis, heart disease, etc. Before going on to discuss the therapeutic potential of molecular compounds of selenium and tellurium, a comment on tellurium toxicity is warranted.
Tellurium is generally thought to be more toxic than selenium but, of course this is dependent on the form of the element.3b,f Tellurium-tolerant fungi are known to incorporate tellurium into sulphur-containing amino acids to form tellurocysteine and telluromethionine.3g In a recent and broad ranging overview of the biological activities of tellurium compounds,3h it was pointed out that tellurium may face the same prejudice as selenium once did and that natural biological functions for tellurium may be revealed in time. Also salient was the observation that tellurium–carbon bonds are more labile than their selenium counterparts and so bond cleavage occurs much more readily, a possible explanation of why tellurium-containing amino acids have yet to be observed naturally.3g
With this background in mind, it is the therapeutic potential of selenium and tellurium against a variety of human ailments (with the exception of cancer) which forms the focus of this Frontier.
Drug development of selenium-containing molecules
Given the multiple biological roles of selenium, perhaps it is not so surprising that efforts have been directed towards the evaluation of synthetic selenium compounds for pharmaceutical applications. Highlights of progress made since the seminal review of Mugesh, du Mont and Sies,4 are included herein, motivated by the desire to highlight the range of selenium compounds and the targeted diseases under investigation.Stimulated by the presence of selenocysteine in the anti-oxidant glutathione peroxidase, there has been active research seeking synthetic selenium-containing anti-oxidants, capable of reducing hydrogen peroxide and other hydroperoxides.2i,4,5a Arguably, the compound attracting most interest and sparking further research is the glutathione peroxidase mimic Ebselen (2-phenyl-1,2-benzoselenazol-3-one), Scheme 1, a compound for which a synthesis was first described in 1924.5b Ebselen is able to catalyse the inactivation of hydroperoxides not only by using glutathione as a substrate, but utilising a variety of other thiols. Complementing this function is the ability of Ebselen to reduce peroxynitrite (ONOO−) to nitrite, diminishing harm done to host cellular constituents when the concentration of peroxynitrite is too high. The putative therapeutic benefits, e.g. as an anti-inflammatory agent, of Ebselen are hampered by insolubility leading to the quest for second-generation Ebselen agents, with design strategies often based on what is known about the catalytic mechanism (Scheme 1);5c,d the sulphur analogue of Ebselen (1) is inactive pointing to the key role of selenium.5a
 | ||
| Scheme 1 Working catalytic cycle for Ebselen (1), adapted from ref. 2i. | ||
Ebselen (1) is thought to readily react with the thiol co-substrate to afford the ring-opened product after Se–N bond cleavage (2). The rate-determining step is disproportionation leading to the di-selenide (3) which reacts with H2O2 to produce the selenenic acid (4) or the seleninic acid, (5). The latter reacts with thiol to generate (4). Regeneration of (1) from (4) can occur via a dehydration reaction or the catalytic cycle can continue by-passing (1), via the reaction of (4) with thiol and dehydration.
Three classes of selenium compounds dominate the search for effective analogues of Ebselen (1), namely (i) cyclic selenenyl amides featuring Se–N bonds, (ii) mononuclear organoselenium compounds having aryl or alkyl substituents, and (iii) diaryl di-selenides. Molecular structures established by X-ray crystallography6a–d of exemplars of (i)–(iii) as well as that of Ebselen are illustrated in Fig. 1.
 | ||
| Fig. 1 Molecular structures of Ebselen (1) and analogues as established by X-ray crystallography: (a) Ebselen, (b) three Ebselen molecules on a benzene ring scaffold, (c) an organometallic selenium compound, and (d) a diaryl di-selenide. | ||
Structure/activity studies suggest that the following features are required for efficacy: (i) a stable Se–C(aromatic) bond for protection against the release of selenium to minimize toxic side-effects, (ii) an Se–N bond to ensure glutathione peroxidase-like activity, and (iii) the presence of a N–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) bond for stabilisation of the selenamide intermediates in the catalytic pathway.5a
O) bond for stabilisation of the selenamide intermediates in the catalytic pathway.5a
Other classes of selenium compounds have been evaluated for their anti-inflammatory activity based, in part, on experimental findings that 1,4-phenylene-bis(methylene)selenocyanate, (6), is capable of affecting cyclooxygenase-2 (COX-2) expression by inactivating the redox-active transcription factor, NF-κB.7a Notable examples that have been investigated are selenium derivatives of the COX-2 inhibitor Celecoxib (7),7b where selenium has been inserted into the molecule by replacing the CF3 group with CH2SeCN, i.e. (8), and by inserting SeCH2 into a C–H bond of the methyl substituent i.e. (9) (see Scheme 2). The expectation was that enhanced therapeutic benefits would manifest from the combination of inhibitory behaviour of Celecoxib coupled with the preventative action of selenium.7b As a proof of concept, (8) was demonstrated to be more potent in macrophages than either (6) or (9) in inhibiting COX-2. This potency was accompanied by the observation that (8) also significantly moderated COX-2 protein expression and suppressed NF-κB activation, an observation correlated with the ability of (8) to extrude small amounts of selenium.7b Related studies of the antinociceptive potential of di-selenides such as diphenyl di-selenide are on-going.7c
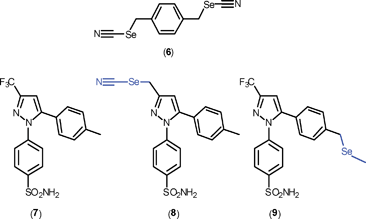 | ||
| Scheme 2 Chemical structures of 1,4-phenylenebis(methylene)-selenocyanate (6), Celecoxib (7), and selenium derivatives of Celecoxib derivatised at the original CF3 group, (8), and derivatised at the original CH3 group, (9). | ||
Selenium is associated with the functioning of the thyroid gland and selenium analogues of anti-thyroid drugs continue to attract attention.8a Selenium deficiency is implicated in chronic autoimmune thyroiditis with regular dietary levels of selenium required for thyroid hormone synthesis and as a defence against excessive levels of iodine. Further, and as mentioned above, selenocysteine is found in the active catalytic sites of the iodothyronine deiodinases. While still under investigation,8b the key role of the selenium (as a selenol? a selenoate?) in the iodothyronine deiodinases and other enzymes, such as lactoperoxidase, is to facilitate the deiodination of thyroxine (10), Scheme 3, to form a selenenyl iodide intermediate that subsequently reacts with an unidentified thiol cofactor to close the catalytic cycle.8b The investigation of the therapeutic role of selenium compounds arises from the known ability of thioamide derivatives such as the drugs 6-n-propyl-2-thiouracil (11) and 1-methyl-3H-imidazole-2-thione (methimazole) (12) to curtail the deiodination of thyroxine and/or the synthesis of thyroid hormone. These drugs find use in combating hyperthyroidism which occurs due to the over-production of thyroid-stimulating hormone. The mechanism of action of the established thioamide drugs is uncertain and subject to some debate, with proposals ranging from their ability to react with or coordinate the iron centre in the thyroid peroxidase enzyme, to their competition reactions with other species for the oxidised form of iodide.8b
 | ||
| Scheme 3 Chemical structures of thyroxine (10), 6-n-propyl-2-thiouracil (11) and methimazole (12), selenium derivatives of (12): 1-methyl-3H-imidazole-2-selenone (13), its zwitterionic form (13z) and di-seleno derivative (14). | ||
A particular motivation for the evaluation of selenium analogues of (11) and (12) relates to the greater nucleophilicity of selenium compared to sulphur.8c Thus, the selenium analogues were expected to react with the selenenyl iodide intermediate to generate a di-selenide in preference to the putative species with a Se–S bond.8c Militating this would be the expected straightforward reduction of the di-selenide by thiols, such as glutathione. Further, selenium-containing molecules might be expected to reduce H2O2 owing to their established anti-oxidant credentials (see above).8d The selenium analogue of (12), i.e. 1-methyl-3H-imidazole-2-selenone (13), proves to be an effective and reversible in vitro inhibitor of oxidation and iodination reactions catalysed by lactoperoxidase. The mechanism is likely to involve the reduction of H2O2 (which is otherwise required for the oxidation of the iron centre in the enzyme) akin to the anti-oxidant ability of glutathione peroxidase, rather than a direct reaction with the enzyme itself. It is noted that (12) and related thioamides such as (11), with their high anti-thyroid activity, are known to exist primarily in its thione form, Scheme 3, precluding their oxidation to the corresponding disulfide, a characteristic which is important for the inhibition of the in vivo synthesis of thyroid hormone.8e Crucially, the behaviour of (13) is quite distinct to that of (12). The di-selenide formed upon oxidation of (13) is readily generated, an attribute that is ascribed to the significant contribution of its zwitterionic form, (13z) in Scheme 3, to the overall electronic structure. In vivo, thiols, e.g. glutathione, will presumably reduce the di-selenide to generate the highly reactive, formally charged nucleophile (13z).8b
The propensity of this class of compounds to have significant residual negative charge on the selenium atoms with concomitant enhanced nucleophilicity has been proposed to explain the greater inhibition potential of compounds related to (13), e.g. (14), towards peroxynitrite- and lactoperoxidase-promoted nitration of protein tyrosine residues compared to their sulfur-containing counterparts.8f
Ebselen (1), ebselen analogues and di-selenides have been investigated for their anti-microbial and anti-viral activities.9 Quite often, these proved to have moderate activity only and to be toxic, but exceptions have been found. The simple di-selenide PhSeSePh proved more effective than derivatives against a panel of 116 pathogenic fungi with greatest inhibitory activity evident against Candida albicans, Candida dubliniensis, Aspergillus spp. and Fusarium spp.9c Comparative studies indicated greater potency for gram-positive bacteria strains over gram-negative bacteria.9d Evolving drug resistance has prompted many anti-microbial studies, including for infections caused by Staphylococcus aureus. The R-enantiomer of compound (15), Scheme 4, is a selenium analogue of an effective anti-staphylococcal drug where the thiophene-ring-sulfur has been replaced by selenium;9e the original drug functions as a topoisomerase poison. Of the selenium compounds evaluated, non-cytotoxic R-(15), was more potent than its sulfur analogue.9e In a related application, thin-films containing Ebselen (1) have been fabricated as surface coatings for biological implants such as catheters.9f Anti-viral screening of selenium compounds is more limited but points to the efficacy of having selenium present and especially to the potential of di-selenides.9g Against an in vitro panel comprising three virus types: Human herpes virus type 1 (HHV-1), Encephalomyocarditis virus (EMCV) and Vesicular stomatitis virus (VSV), selenium compounds displayed efficacy against HHV-1 and EMCV but, not against VSV.9g The most effective compound was the di-selenide (16), Scheme 4. When the selenium was replaced by sulfur, no activity was evident.9g An indication that selenium substitution for sulfur is not a new drug panacea is found in the in vitro anti-HIV screening results for a series of 1,2,3-selenadiazole thioacetanilides, represented by (17) in Scheme 4.9h This substitution of sulfur by selenium in (17) did not result in enhanced inhibition of in vitro HIV-1 replication but did result in greater cytotoxicity.9h
![Chemical structures of an effective anti-staphylococcal agent (15), of an anti-viral agent N-ethyl-2-{[2-(ethylcarbamoyl)phenyl]-diselanyl}benzamide (16), of N-(4-Acetyl-2-bromophenyl)-2-(4-(3,4-dichlorophenyl)-1,2,3-selenadiazol-5-ylthio)acetamide (17) trialled for anti-HIV activity, of a potential anti-Leishmaniasis agent 4-[(4-aminophenyl)diselanyl]aniline (18), and of anti-parasite compound 4-(1,4-dioxonaphthalen-2-yl)-N-[3-(phenylselanyl)propyl]butanamide (19).](/image/article/2012/DT/c2dt12225a/c2dt12225a-s4.gif) | ||
| Scheme 4 Chemical structures of an effective anti-staphylococcal agent (15), of an anti-viral agent N-ethyl-2-{[2-(ethylcarbamoyl)phenyl]-diselanyl}benzamide (16), of N-(4-Acetyl-2-bromophenyl)-2-(4-(3,4-dichlorophenyl)-1,2,3-selenadiazol-5-ylthio)acetamide (17) trialled for anti-HIV activity, of a potential anti-Leishmaniasis agent 4-[(4-aminophenyl)diselanyl]aniline (18), and of anti-parasite compound 4-(1,4-dioxonaphthalen-2-yl)-N-[3-(phenylselanyl)propyl]butanamide (19). | ||
The potential of selenium compounds as a new therapy for the treatment of Leishmaniasis has also been explored.9i Selenocyanates and di-selenides also displayed potency against Leishmania infantum promastigotes (in the sandfly host) and effective derivatives were further evaluated for efficacy against a model for amastigotes (in the infected human). It was a non-cytotoxic di-selenide, (18) in Scheme 4, that exhibited the greatest potency amongst the compounds investigated.9i Featuring a quinone residue, also known for its ability to function as a redox modulator, as well as selenium, it was not surprising that compound (19) inhibited Plasmodium falciparum, the causative agent of malaria.9j
While not strictly molecular compounds, given the emergence of nanotechnology for a variety of medicinal applications, for completeness, a brief mention will be made of the therapeutic potential of elemental selenium. Building upon the known anti-fungal activity of selenium sulfide, found in anti-dandruff formulations,10a selenium nanoparticles generated biogenically by Klebsiella pneumoniae (average size 200–300 nm), were found to be effective anti-fungal agents against different species of Aspergillus and Malassezia.10b When dissolved in DMSO at doses of 100 μg kg−1, elemental selenium was found to produce a curative effect in indomethacin-induced gastric ulcers in rats.10c Finally, the incorporation of selenium in ionic liquids has been recently reported to result in materials that exhibit anti-microbial activity, depending on substitution patterns.10d Ionic liquid (20), Scheme 5, with chloride as the counter-ion was shown to be effective against an algae, Prototheca zopfii, whereas the xylyl derivative (21), also as a chloride, was the most effective against Staphylococcus aureus. The influence of selenium and the counter-ion (i.e. Cl−, BF4− or PF6−) were ascertained by comparing the anti-algae activities of (22) with the selenium-containing counterparts. The selenium analogues were uniformly more active than (22), and of the selenium-containing ionic liquids, using chloride as the counter-ion resulted in the highest activity.10d
![Chemical structures of selected ionic ligands featuring 3-methyl-1-{[(2,4,6-trimethylphenyl)selanyl]methyl}imidazol-1-ium (20), 1-{[(4-chlorophenyl)selanyl]methyl}-3-methylimidazol-1-ium (21), and 3-methyl-1-(2-phenylethyl)imidazol-1-ium (22) cations. The anions, X−, are Cl−, BF4− and PF6− in each case, and play a role in biological efficacy.](/image/article/2012/DT/c2dt12225a/c2dt12225a-s5.gif) | ||
| Scheme 5 Chemical structures of selected ionic ligands featuring 3-methyl-1-{[(2,4,6-trimethylphenyl)selanyl]methyl}imidazol-1-ium (20), 1-{[(4-chlorophenyl)selanyl]methyl}-3-methylimidazol-1-ium (21), and 3-methyl-1-(2-phenylethyl)imidazol-1-ium (22) cations. The anions, X−, are Cl−, BF4− and PF6− in each case, and play a role in biological efficacy. | ||
Drug development of tellurium-containing molecules
Knowledge of the anti-microbial activity of tellurium, in the form of potassium tellurite, 2 K+ TeO32−, dates back over a century.11a Sir Alexander Fleming reported tellurite was active in penicillin-insensitive bacteria and that penicillin was active in tellurium-insensitive bacteria.11b Despite these early beginnings, with a few notable exceptions, further research into the potential of tellurium as an antibiotic or other medicine has not attracted significant attention. This is perhaps regrettable,3h,11a given the wide-ranging biological activities exhibited by the non-toxic potent immunomodulator ammonium trichloro(dioxoethylene)tellurate(IV), (23), Scheme 6.11c Owing to its anti-tumour activity, clinical trials have been performed on (23).11d Subsequently, (23) was shown to exhibit potential against Parkinson's11e and auto-immune disease,11f and topical formulations show promise for the treatment of dermatitis.11g Compound (23)11g and others, e.g. binuclear (24),11h exhibit anti-viral activity with (24) selectively inhibiting cysteine proteases.11h![Chemical structures of non-toxic and potent immunomodulator trichloro(dioxoethylene)tellurate(IV) anion {ammonium ion not shown}, (22), of anti-viral bis((R,R)-tartrate)-di-tellurium(iv) (23), of an anti-epileptic (3E)-2-chloro-3-(chloromethylidene)-2-(4-methoxyphenyl)-1-oxa-2λ4-tellura-spiro[3.5]nonane {ammonium ion not shown} (24), anti-malarial 3-(butyltellanyl)propane-1-sulfonate (25), anti-Leishmanial telluride, 2,2,2,4-tetrachloro-2,5-dihydro-1,2λ4-oxatellurol-2-uide (26), and anti-oxidants diethyl [(Z)-2-phenyl-2-(phenyltellanyl)ethenyl]phosphonate (27) and [(Z)-1-(butyltellanyl)-2-(methylsulfanyl)ethenyl]benzene (28).](/image/article/2012/DT/c2dt12225a/c2dt12225a-s6.gif) | ||
| Scheme 6 Chemical structures of non-toxic and potent immunomodulator trichloro(dioxoethylene)tellurate(IV) anion {ammonium ion not shown}, (22), of anti-viral bis((R,R)-tartrate)-di-tellurium(IV) (23), of an anti-epileptic (3E)-2-chloro-3-(chloromethylidene)-2-(4-methoxyphenyl)-1-oxa-2λ4-tellura-spiro[3.5]nonane {ammonium ion not shown} (24), anti-malarial 3-(butyltellanyl)propane-1-sulfonate (25), anti-Leishmanial telluride, 2,2,2,4-tetrachloro-2,5-dihydro-1,2λ4-oxatellurol-2-uide (26), and anti-oxidants diethyl [(Z)-2-phenyl-2-(phenyltellanyl)ethenyl]phosphonate (27) and [(Z)-1-(butyltellanyl)-2-(methylsulfanyl)ethenyl]benzene (28). | ||
Organotellurium compounds have also been investigated sporadically for pharmacological potential with (25) demonstrating anti-convulsant effects correlated with its ability to inhibit cysteine-containing caspases.12a Thiol-containing low Mr thioredoxin reductases were the targets for molecules such as sulfonic acid derived (26), which proved effective in trials for anti-malarial activity.12b In this context, the tellurium analogue of (19), Scheme 4, was marginally more effective than (19) against Plasmodium falciparum.9j The organotellurium anion in (27) appeared effective against both the flagellate and non-flagellate parasite forms in an in vivo model for Leishmaniasis.12c Finally, exploiting the known anti-oxidant activity of tellurium,12d vinyltellurium derivatives such as (28)12e and (29)12f have proved to possess excellent anti-oxidant abilities with potential against excitotoxic agents.
Conclusions
Complementing natural biological functions, selenium compounds are emerging as potential therapeutic agents. Many of these applications rely on the inherent anti-oxidant ability of selenium, but not exclusively. Thus, while molecular selenium compounds demonstrate efficacy against inflammatory diseases and can combat hyperthyroidism, they also exhibit anti-microbial and anti-viral activities. Compared with selenium, tellurium is clearly lagging in terms of drug development. Perceived problems with toxicity may have hampered investigations but, with the inclusion of toxic elements such as arsenic and platinum into the pharmacopoeia, tellurium compounds administered in an appropriate dose regime may yet prove efficacious. Certainly, both selenium and tellurium compounds exhibit a range of biological profiles and both are deserving of more attention.Acknowledgements
The Ministry of Higher Education (Malaysia) is thanked for funding studies in Metal-Based Drugs through the High-Impact Research scheme (UM.C/HIR/MOHE/SC/12).Notes and references
- (a) M. Gielen and E. R. T. Tiekink in Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine, eds M. Gielen and E. R. T. Tiekink, John Wiley & Sons Ltd, Chichester, 2005, pp. 421–439 Search PubMed; (b) B. Yuan, Y. Yoshino, T. Kaise and H. Toyoda in Biological Chemistry of Arsenic, Antimony and Bismuth, ed. H. Sun, Wiley, Chichester, 2011, pp. 263–292 Search PubMed; (c) N. Yang and H.-Z. Sun, in Biological Chemistry of Arsenic, Antimony and Bismuth, ed. H. Sun, Wiley, Chichester, 2011, pp. 53–81 Search PubMed; (d) A. H. W. Mendis and B. J. Marshall in Biological Chemistry of Arsenic, Antimony and Bismuth, ed. H. Sun, Wiley, Chichester, 2011, pp. 241–262 Search PubMed.
- (a) L. Flohe and Biochim, Biochim. Biophys. Acta, Gen. Subj., 2009, 1790, 1389 CrossRef CAS; (b) T. Řezanka and K. Sigler, Phytochemistry, 2008, 69, 585 CrossRef; (c) J. Pinset, Biochem. J., 1954, 57, 10 Search PubMed; (d) D. L. Klayman and W. H. H. Gunter, Organic Selenium Compounds-Their Chemistry and Biology, Wiley, New York, 1973 Search PubMed; (e) R. A. Zingaro and W. C. Cooper, Selenium, van Nostrand Reinhold Company, New York, 1974 Search PubMed; (f) D. C. Turner and T. C. Stadtman, Arch. Biochem. Biophys., 1973, 154, 366 CrossRef CAS; (g) L. Flohe, W. A. Gunzler and H. H. Schock, FEBS Lett., 1973, 32, 132 CrossRef CAS; (h) J. Chambers, J. Frampton, P. Goldfarb, N. Affara, W. McBain and P. R. Harrison, EMBO J, 6, 1221 Search PubMed; (i) K. P. Bhabak and G. Mugesh, Acc. Chem. Res., 2010, 43, 1408 CrossRef CAS; (j) S. Antony and C. A. Bayse, Inorg. Chem., 2011, 50, 12075 CrossRef CAS; (k) M. Mobli, D. Morgenstern, G. F. King, P. F. Alewood and M. Muttenthaler, Angew. Chem., Int. Ed., 2011, 50, 11952 CrossRef CAS; (l) V. Nascimento, E. E. Alberto, D. W. Tondo, D. Dambrowski, M. R. Detty, F. Nome and A. L. Braga, J. Am. Chem. Soc., 2012, 134, 138 CrossRef CAS; (m) I. Carcelli, J. Zukerman-Schpector and E. R. T. Tiekink, Coord. Chem. Rev., 2012, 256, 412 CrossRef; (n) S. E. Jackson-Rosario and W. T. Self, Metallomics, 2010, 2, 112 RSC.
- (a) S. W. May, Expert Opin. Invest. Drugs, 2002, 11, 1261 CrossRef CAS; (b) C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255 CrossRef CAS; (c) M. Birringer, S. Pilawa and L. Flohé, Nat. Prod. Rep., 2002, 19, 693 RSC; (d) S. J. Fairweather-Tait, T. Bao, M. R. Broadly, R. Collings, D. Ford, J. E. Hesketh and R. Hurst, Antioxidants Redox Signalling, 2011, 14, 1338 Search PubMed; (e) L. Fohlé, in Selenium and Tellurium Chemistry, eds J. D. Woollins and R. S. Laitinen, Springer-Verlag, Berlin Heidleberg, 2011, pp. 285–302 Search PubMed; (f) L. Engman, Acc. Chem. Res., 1985, 18, 274 CrossRef CAS; (g) S. E. Ramadan, A. A. Razak, A. M. Ragab and M. El-Meleigy, Biol. Trace Elem. Res., 1989, 20, 225 CrossRef CAS; (h) R. L. O. R. Cunha, I. E. Gouvea and L. Juliano, An. Acad. Bras. Cienc., 2009, 81, 393 CrossRef CAS.
- G. Mugesh, W.-W. Du Mont and H. Sies, Chem. Rev., 2001, 101, 2125 CrossRef CAS.
- (a) M. Ninomiya, D. R. Garud and M. Koketsu, Coord. Chem. Rev., 2011, 255, 2968 CrossRef CAS; (b) R. Lesser and R. Weiss, Ber. Dtsch. Chem. Ges., 1924, 57, 1077 CrossRef; (c) B. K. Sarma and G. Mugesh, J. Am. Chem. Soc., 2005, 127, 11477 CrossRef CAS; (d) K. P. Bhabak and G. Mugesh, Chem.–Asian J., 2009, 4, 974 CrossRef CAS.
- (a) L. Dupont, O. Dideberg and P. Jacquemin, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1990, 46, 484 CrossRef; (b) K. P. Bhabak and G. Mugesh, Chem.–Eur. J., 2007, 13, 4594 CrossRef CAS; (c) B. J. Bhuyan and G. Mugesh, Org. Biomol. Chem., 2011, 9, 1356 RSC; (d) B. K. Sarma, D. Manna, M. Minoura and G. Mugesh, J. Am. Chem. Soc., 2010, 132, 5364 CrossRef CAS.
- (a) K. El-Bayoumy, A. Das, B. Narayanan, N. Narayanan, E. S. Fiala, D. Desai, C. V. Rao, S. Amin and R. Sinha, Carcinogenesis, 2006, 27, 1369 CrossRef CAS; (b) D. Desai, N. Kaushal, U. H. Gandi, R. J. Arner, C. D'Souza, G. Chen, H. Vunta, K. El-Bayoumy, S. Amin and K. S. Prabhu, Chem.-Biol. Interact., 2010, 188, 446 CrossRef CAS; (c) L. Savegnago, L. G. Pinto, C. R. Jesse, D. Alves, J. B. T. Rocha, C. W. Nogueira and G. Zeni, Eur. J. Pharmacol., 2007, 555, 129 CrossRef CAS.
- (a) G. Roy, M. Nethaji and G. Mugesh, Org. Biomol. Chem., 2006, 4, 2883 RSC; (b) G. Roy and G. Mugesh, Chem. Biodiversity, 2008, 5, 414 CrossRef CAS; (c) A. Taurog, M. L. Dorris, L. J. Guziec and F. S. Guziec Jr, Biochem. Pharmacol., 1994, 48, 1447 CrossRef CAS; (d) G. Roy and G. Mugesh, J. Am. Chem. Soc., 2005, 127, 15207 CrossRef CAS; (e) G. J. Corban, S. K. Hadjikakou, N. Hadjiliadis, M. Kubicki, E. R. T. Tiekink, I. S. Butler, E. Drougas and A. M. Kosmas, Inorg. Chem., 2005, 44, 8617 CrossRef CAS; (f) K. P. Bhabak, K. Satheeshkumar, S. Jayavelu and G. Mugesh, Org. Biomol. Chem., 2011, 9, 7342 Search PubMed.
- (a) Sh. H. Abdel-Hafez, Phosphorus, Sulfur Silicon Relat. Elem., 2010, 185, 2543 CrossRef CAS; (b) Sh. H. Abdel-Hafez, Russ. J. Bioorg. Chem., 2010, 36, 370 CrossRef CAS; (c) E. S. Loreto, D. A. N. Mario, J. M. Santurio, S. H. Alves, C. W. Nogueira and G. Zeni, Mycoses, 2011, 54, e572 CrossRef; (d) M. Piętka-Ottlik, H. Wójtowicz-Młochowska, K. Kołodziejczyk, E. Piasecki and J. Młochowski, Chem. Pharm. Bull., 2008, 56, 1423 CrossRef; (e) J. A. Wiles, A. S. Phadke, B. J. Bradbury, M. J. Pucci, J. A. Thanassi and M. Deshpande, J. Med. Chem., 2011, 54, 3418 CrossRef CAS; (f) W. Cai, J. Wu, C. Xi, A. J. Ashe III and M. E. Meyerhoff, Biomaterials, 2011, 32, 7774 CrossRef CAS; (g) M. Pietka-Ottlik, P. Potaczek, E. Piasecki and J. Mlochowski, Molecules, 2010, 15, 8214 CrossRef CAS; (h) P. Zhan, X. Liu, Z. Fang, C. Pannecouque and E. De Clerq, Bioorg. Med. Chem., 2009, 17, 6374 CrossRef CAS; (i) D. Plano, Y. Baquedano, D. Moren-Mateos, M. Font, A. Jiménez-Ruiz, J. A. Palop and C. Sanmartin, Eur. J. Med. Chem., 2011, 46, 3315 CrossRef CAS; (j) S. Mecklenburg, S. Shaaban, L. A. Ba, T. Burkholz, T. Schneider, B. Diesel, A. K. Kiemer, A. Röseler, K. Becker, J. Reichrath, A. Stark, W. Tilgen, M. Abbas, L. A. Wessjohann, F. Sasse and C. Jacob, Org. Biomol. Chem., 2009, 7, 4753 RSC.
- (a) J. Van Cutsem, F. Van Gerven, J. Frasen, P. Schrooten and P. A. Janssen, J. Am. Acad. Dermatol., 1990, 22, 993 CrossRef CAS; (b) A. R. Shahverdi, A. Fakhimi, G. Mosavat, P. Jafari-Fesharaki, S. Rezaie and S. M. Rezayat, World Appl. Sci. J., 2010, 10, 918 CAS; (c) J.-H. Kim, B.-W. Kim, H.-J. Kwon and S.-W. Nam, J. Microbiol. Biotechnol., 2011, 21, 400 CAS; (d) E. A. Alberto, L. L. Rossato, S. H. Alves, D. Alves and A. L. Braga, Org. Biomol. Chem., 2011, 9, 1001 RSC.
- (a) L. A. Ba, M. Döring, V. Jamier and C. Jacob, Org. Biomol. Chem., 2010, 8, 4203 RSC; (b) A. Fleming, J. Pathol. Bacteriol., 1932, 35, 831 CrossRef CAS; (c) B. Sredni, R. R. Caspi, A. Klein, Y. Kalechman, Y. Danziger, M. Benyaakov, T. Tamari, F. Shalit and M. Albeck, Nature, 1987, 330, 173 CrossRef CAS; (d) G. M. Frei, M. Kremer, K.-M. Hanschmann, S. Krause, M. Albeck, B. Sredni and B. S. Schnierle, Br. J. Dermatol., 2008, 158, 578 CrossRef CAS; (e) B. Sredni, R. Geffen-Aricha, W. Z. Duan, M. Albeck, F. Shalit, H. M. Lander, N. Kinor, O. Sagi, A. Albeck, S. Yosef, M. Brodsky, D. Sredni-Kenigsbuch, T. Sonino, D. L. Longo, M. P. Mattson and G. Yadid, FASEB J., 2007, 21, 1870 CrossRef CAS; (f) Y. Kalechman, U. Gafter, J. P. Da, M. Albeck, D. Alarcon-Segovia and B. Sredni, J. Immunol., 1997, 159, 2658 CAS; (g) D. Sredni-Kenigsbuch, M. Shohat, B. Shohat, D. Ben-Amitai, C.-C. Chan and M. David, J. Immunol., 2007, 178, 37.3 Search PubMed; (h) B. Sredni, Z. Rosenthal-Galili, H. Michlin, D. Sobelman, Y. Seger, S. Blagerman, Y. Kalechman and B. Rager-Zisman, Immunol. Lett., 1994, 43, 159 CrossRef CAS; (i) S. Yosef, M. Brodsky, B. Sredni, A. Albeck and M. Albeck, ChemMedChem, 2007, 2, 1601 CrossRef CAS.
- (a) D. S. Persike, R. L. O. R. Cunha, L. Juliano, L. R. Silva, F. E. Rosim, T. Vignoli, F. Dona, E. A. Cavalheiro and M. J. da Silva Fernandes, Neurobiol. Dis., 2008, 31, 120 CrossRef CAS; (b) P. J. McMillan, E. M. Patzewitz, S. E. Young, G. D. Westrop, G. H. Coombs, L. Engman and S. Mueller, Parasitology, 2008, 136, 27 CrossRef; (c) C. B. C. Lima, W. W. Arrais-Silva, R. L. O. R. Cunha and S. Giorgio, Korean J. Parasitol., 2009, 47, 213 CrossRef CAS; (d) C. Jacob, G. E. Arteel, T. Kanda, L. Engman and H. Sies, Chem. Res. Toxicol., 2000, 13, 3 CrossRef CAS; (e) V. C. Borges, L. Savegnago, S. Pinton, C. R. Jesse, D. Alves and C. W. Nogueria, J. Appl. Toxicol., 2008, 28, 839 CrossRef CAS; (f) D. S. Ávila, P. Gubert, A. Palma, D. Colle, D. Alves, C. W. Nogueira, J. B. T. Rocha and D. A. A. Soares, Brain Res. Bull., 2008, 76, 114 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
